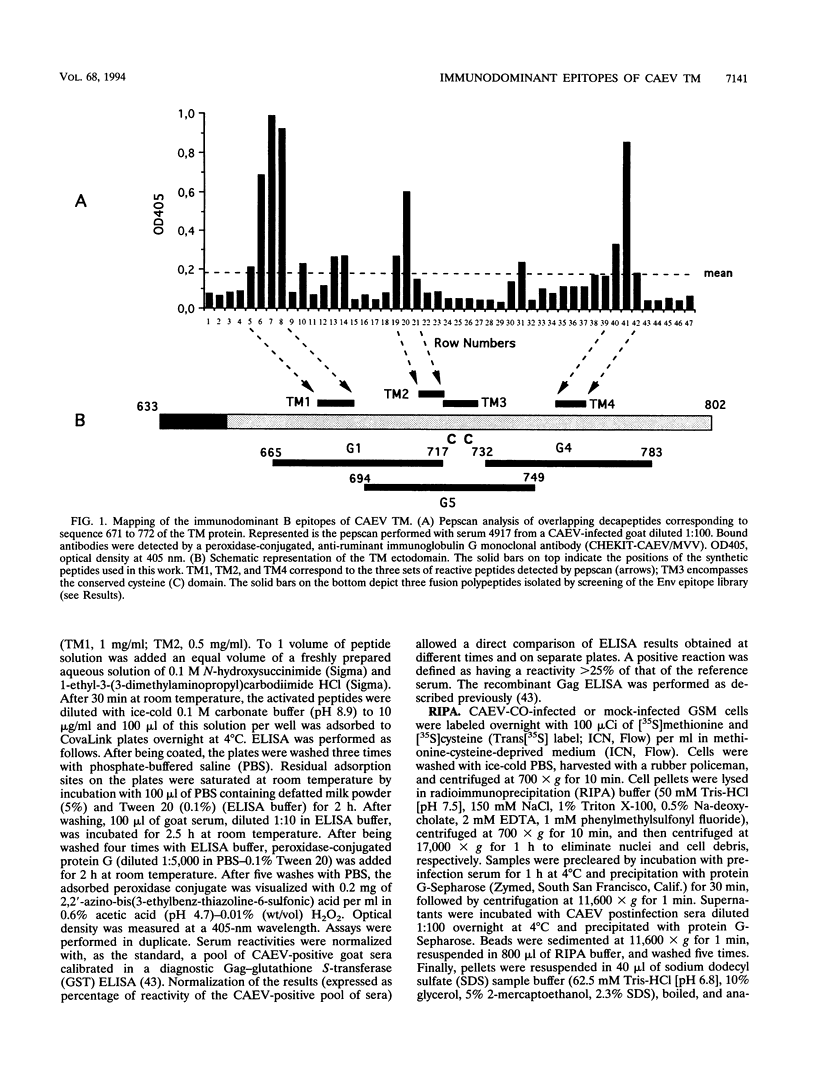
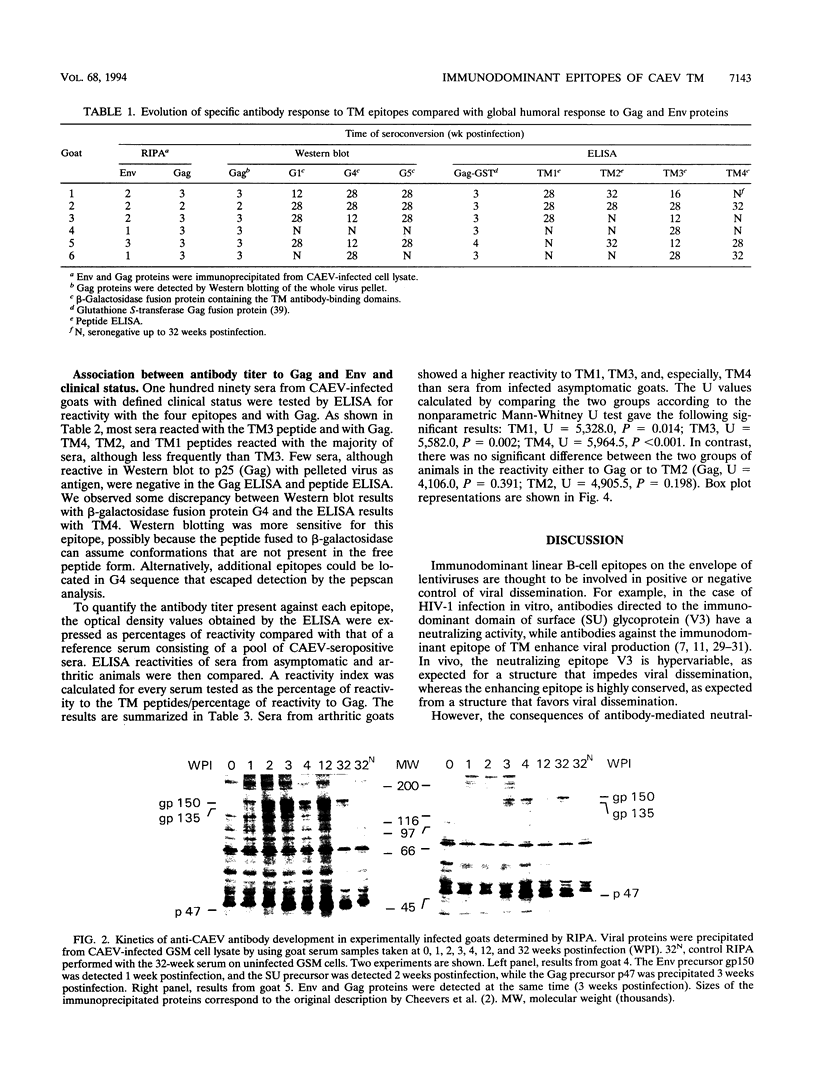
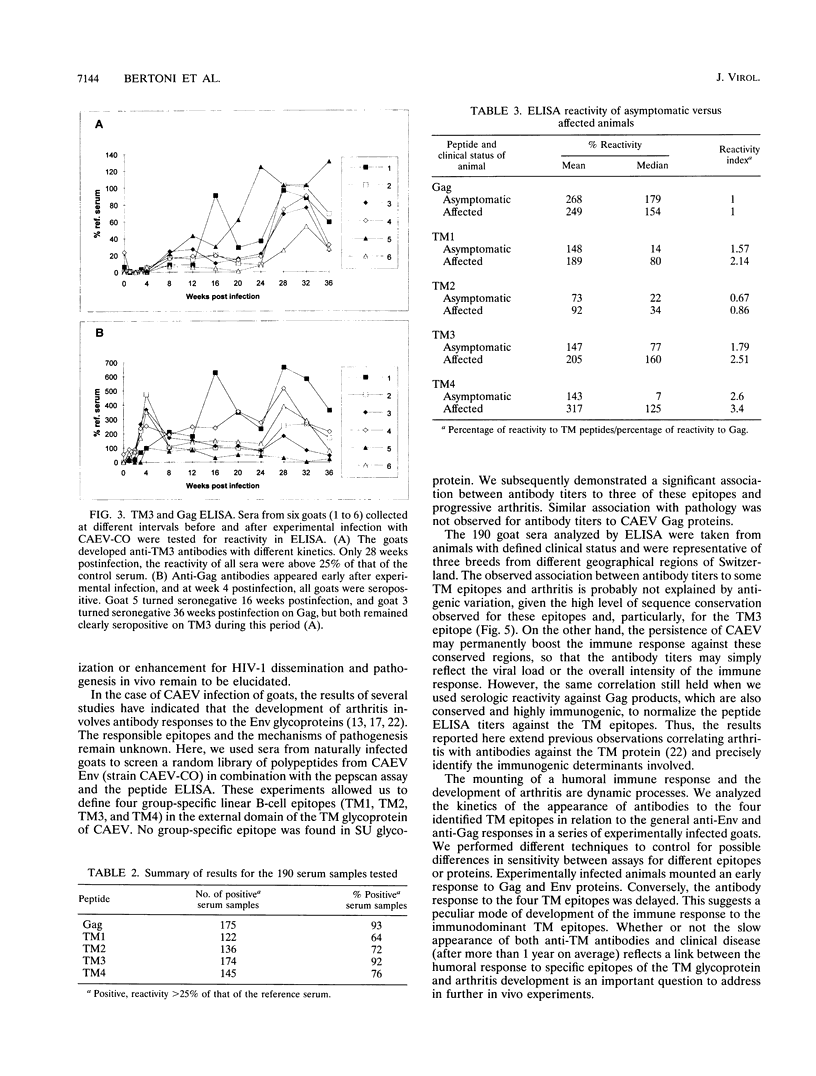
Abstract
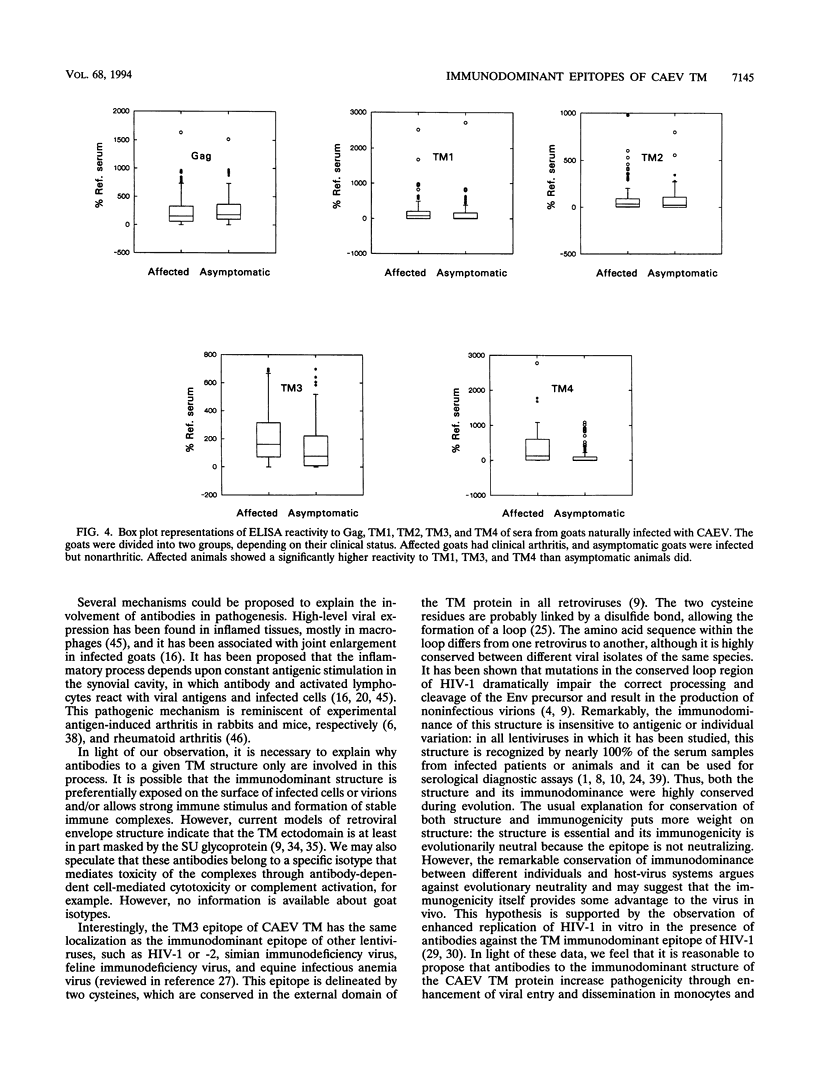
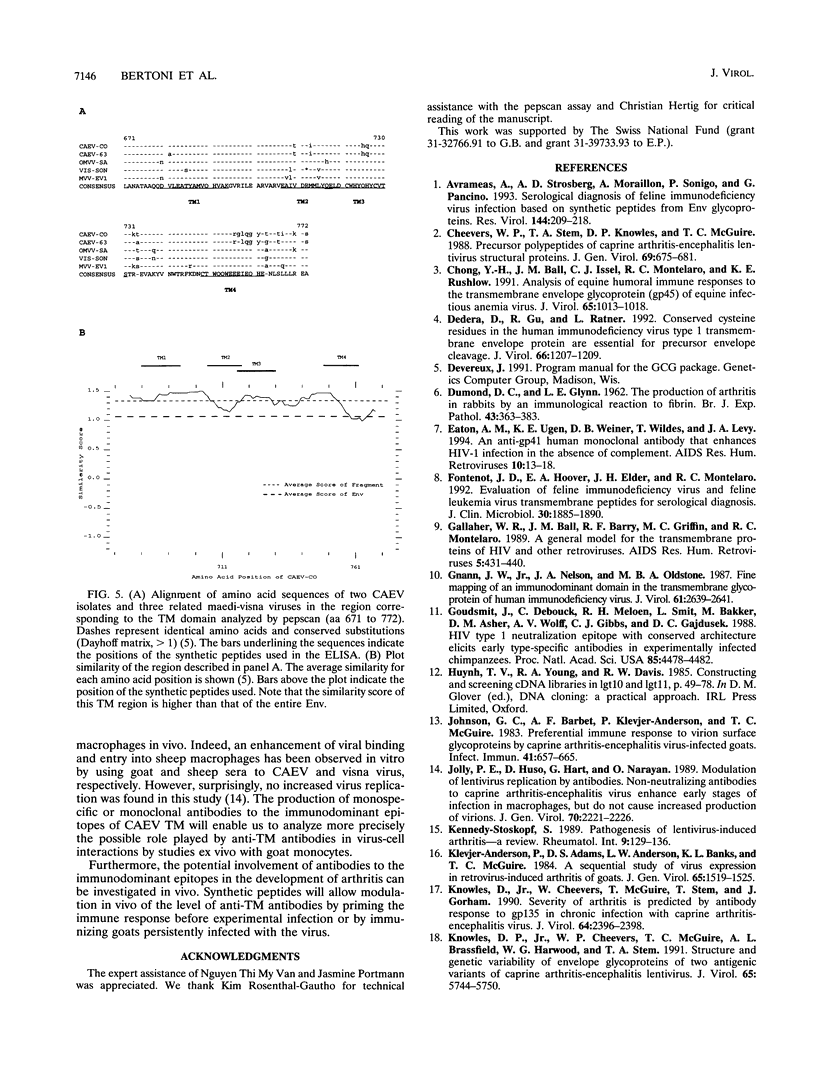
High titers of antibodies to caprine arthritis-encephalitis virus (CAEV) envelope (Env) glycoproteins are found in infected goats developing a progressive arthritis. In order to identify linear B epitopes of the CAEV Env, which may be involved in the immunopathology of arthritis, we constructed a lambda gt11 Env expression library. By combining library screening with sera from naturally infected Swiss goats with an enzyme immunoassay with overlapping peptides (pepscan), four group-specific epitopes could be precisely defined in the transmembrane envelope proteins: TM1 to TM4, including a conserved structure (TM3) that corresponds to the immunodominant epitope of human immunodeficiency virus type 1 and other lentiviruses. A panel of 190 CAEV naturally infected goat serum samples, obtained from animals with defined clinical status, was tested for reactivity to synthetic peptides corresponding to the TM epitopes in an enzyme-linked immunosorbent assay. Antibody reactivity to two epitopes was highly associated (TM3, P = 0.002, and TM4, P < 0.001) with the presence of clinically detectable arthritis. Such an association is absent for anti-Gag antibody. Antibodies to the immunodominant structures of the TM glycoprotein could thus have an important role in the immunopathogenic process leading to disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas A., Strosberg A. D., Moraillon A., Sonigo P., Pancino G. Serological diagnosis of feline immunodeficiency virus infection based on synthetic peptides from Env glycoproteins. Res Virol. 1993 May-Jun;144(3):209–218. doi: 10.1016/s0923-2516(06)80031-2. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Stem T. A., Knowles D. P., McGuire T. C. Precursor polypeptides of caprine arthritis-encephalitis lentivirus structural proteins. J Gen Virol. 1988 Mar;69(Pt 3):675–681. doi: 10.1099/0022-1317-69-3-675. [DOI] [PubMed] [Google Scholar]
- Chong Y. H., Ball J. M., Issel C. J., Montelaro R. C., Rushlow K. E. Analysis of equine humoral immune responses to the transmembrane envelope glycoprotein (gp45) of equine infectious anemia virus. J Virol. 1991 Feb;65(2):1013–1018. doi: 10.1128/jvi.65.2.1013-1018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Dedera D., Gu R. L., Ratner L. Conserved cysteine residues in the human immunodeficiency virus type 1 transmembrane envelope protein are essential for precursor envelope cleavage. J Virol. 1992 Feb;66(2):1207–1209. doi: 10.1128/jvi.66.2.1207-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton A. M., Ugen K. E., Weiner D. B., Wildes T., Levy J. A. An anti-gp41 human monoclonal antibody that enhances HIV-1 infection in the absence of complement. AIDS Res Hum Retroviruses. 1994 Jan;10(1):13–18. doi: 10.1089/aid.1994.10.13. [DOI] [PubMed] [Google Scholar]
- Fontenot J. D., Hoover E. A., Elder J. H., Montelaro R. C. Evaluation of feline immunodeficiency virus and feline leukemia virus transmembrane peptides for serological diagnosis. J Clin Microbiol. 1992 Jul;30(7):1885–1890. doi: 10.1128/jcm.30.7.1885-1890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R., Ball J. M., Garry R. F., Griffin M. C., Montelaro R. C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989 Aug;5(4):431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Nelson J. A., Oldstone M. B. Fine mapping of an immunodominant domain in the transmembrane glycoprotein of human immunodeficiency virus. J Virol. 1987 Aug;61(8):2639–2641. doi: 10.1128/jvi.61.8.2639-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. C., Barbet A. F., Klevjer-Anderson P., McGuire T. C. Preferential immune response to virion surface glycoproteins by caprine arthritis-encephalitis virus-infected goats. Infect Immun. 1983 Aug;41(2):657–665. doi: 10.1128/iai.41.2.657-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly P. E., Huso D., Hart G., Narayan O. Modulation of lentivirus replication by antibodies. Non-neutralizing antibodies to caprine arthritis-encephalitis virus enhance early stages of infection in macrophages, but do not cause increased production of virions. J Gen Virol. 1989 Aug;70(Pt 8):2221–2226. doi: 10.1099/0022-1317-70-8-2221. [DOI] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S. Pathogenesis of lentivirus-induced arthritis. A review. Rheumatol Int. 1989;9(3-5):129–136. doi: 10.1007/BF00271869. [DOI] [PubMed] [Google Scholar]
- Klevjer-Anderson P., Adams D. S., Anderson L. W., Banks K. L., McGuire T. C. A sequential study of virus expression in retrovirus-induced arthritis of goats. J Gen Virol. 1984 Sep;65(Pt 9):1519–1525. doi: 10.1099/0022-1317-65-9-1519. [DOI] [PubMed] [Google Scholar]
- Knowles D. P., Jr, Cheevers W. P., McGuire T. C., Brassfield A. L., Harwood W. G., Stem T. A. Structure and genetic variability of envelope glycoproteins of two antigenic variants of caprine arthritis-encephalitis lentivirus. J Virol. 1991 Nov;65(11):5744–5750. doi: 10.1128/jvi.65.11.5744-5750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles D., Jr, Cheevers W., McGuire T., Stem T., Gorham J. Severity of arthritis is predicted by antibody response to gp135 in chronic infection with caprine arthritis-encephalitis virus. J Virol. 1990 May;64(5):2396–2398. doi: 10.1128/jvi.64.5.2396-2398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J. R., Mathieson B. J., Zack P. M., Walker M. C., Halstead S. B., Burke D. S. Summary report: workshop on the potential risks of antibody-dependent enhancement in human HIV vaccine trials. AIDS Res Hum Retroviruses. 1993 Dec;9(12):1175–1184. doi: 10.1089/aid.1993.9.1175. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Adams D. S., Johnson G. C., Klevjer-Anderson P., Barbee D. D., Gorham J. R. Acute arthritis in caprine arthritis-encephalitis virus challenge exposure of vaccinated or persistently infected goats. Am J Vet Res. 1986 Mar;47(3):537–540. [PubMed] [Google Scholar]
- McGuire T. C., Knowles D. P., Jr, Davis W. C., Brassfield A. L., Stem T. A., Cheevers W. P. Transmembrane protein oligomers of caprine arthritis-encephalitis lentivirus are immunodominant in goats with progressive arthritis. J Virol. 1992 May;66(5):3247–3250. doi: 10.1128/jvi.66.5.3247-3250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C. The immune response to viral antigens as a determinant of arthritis in caprine arthritis-encephalitis virus infection. Vet Immunol Immunopathol. 1987 Dec;17(1-4):465–470. doi: 10.1016/0165-2427(87)90162-0. [DOI] [PubMed] [Google Scholar]
- Narayan O. Lentiviruses are etiological agents of chronic diseases in animals and acquired immunodeficiency syndrome in humans. Can J Vet Res. 1990 Jan;54(1):42–48. [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Chiodi F., Whalley A., Parks E., Nauclér A., Costa C. M., Torstensson R., Biberfeld G. Site-directed enzyme-linked immunosorbent assay with a synthetic simian immunodeficiency virus SIVmac peptide identifying antibodies against the HIV-2 transmembrane glycoprotein. AIDS. 1989 Jan;3(1):17–20. [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Lewicki H., Dyson H. J., Feher V. A., Assa-Munt N., Wright P. E. Mapping the anatomy of the immunodominant domain of the human immunodeficiency virus gp41 transmembrane protein: peptide conformation analysis using monoclonal antibodies and proton nuclear magnetic resonance spectroscopy. J Virol. 1991 Apr;65(4):1727–1734. doi: 10.1128/jvi.65.4.1727-1734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancino G., Chappey C., Saurin W., Sonigo P. B epitopes and selection pressures in feline immunodeficiency virus envelope glycoproteins. J Virol. 1993 Feb;67(2):664–672. doi: 10.1128/jvi.67.2.664-672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancino G., Ellerbrok H., Sitbon M., Sonigo P. Conserved framework of envelope glycoproteins among lentiviruses. Curr Top Microbiol Immunol. 1994;188:77–105. doi: 10.1007/978-3-642-78536-8_5. [DOI] [PubMed] [Google Scholar]
- Querat G., Audoly G., Sonigo P., Vigne R. Nucleotide sequence analysis of SA-OMVV, a visna-related ovine lentivirus: phylogenetic history of lentiviruses. Virology. 1990 Apr;175(2):434–447. doi: 10.1016/0042-6822(90)90428-t. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Gorny M. K., Xu J. Y., Mitchell W. M., Zolla-Pazner S. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol. 1991 Aug;65(8):4169–4176. doi: 10.1128/jvi.65.8.4169-4176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Kawamura T., Lake D., Masuho Y., Mitchell W. M., Hersh E. M. Antibodies to the primary immunodominant domain of human immunodeficiency virus type 1 (HIV-1) glycoprotein gp41 enhance HIV-1 infection in vitro. J Virol. 1990 Nov;64(11):5301–5305. doi: 10.1128/jvi.64.11.5301-5305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltarelli M., Querat G., Konings D. A., Vigne R., Clements J. E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology. 1990 Nov;179(1):347–364. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- Sargan D. R., Bennet I. D., Cousens C., Roy D. J., Blacklaws B. A., Dalziel R. G., Watt N. J., McConnell I. Nucleotide sequence of EV1, a British isolate of maedi-visna virus. J Gen Virol. 1991 Aug;72(Pt 8):1893–1903. doi: 10.1099/0022-1317-72-8-1893. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P., Vignaux F., Traincard F., Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993 Dec;67(12):7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T. F., Jameson B. A., Lopalco L., Siccardi A. G., Weiss R. A., Moore J. P. Conserved structural features in the interaction between retroviral surface and transmembrane glycoproteins? AIDS Res Hum Retroviruses. 1992 Sep;8(9):1571–1580. doi: 10.1089/aid.1992.8.1571. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Søndergård-Andersen J., Lauritzen E., Lind K., Holm A. Covalently linked peptides for enzyme-linked immunosorbent assay. J Immunol Methods. 1990 Jul 20;131(1):99–104. doi: 10.1016/0022-1759(90)90238-q. [DOI] [PubMed] [Google Scholar]
- Wang J. J., Steel S., Wisniewolski R., Wang C. Y. Detection of antibodies to human T-lymphotropic virus type III by using a synthetic peptide of 21 amino acid residues corresponding to a highly antigenic segment of gp41 envelope protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6159–6163. doi: 10.1073/pnas.83.16.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Z., Rushlow K. E., Issel C. J., Cook R. F., Cook S. J., Raabe M. L., Chong Y. H., Costa L., Montelaro R. C. Enhancement of EIAV replication and disease by immunization with a baculovirus-expressed recombinant envelope surface glycoprotein. Virology. 1994 Feb 15;199(1):247–251. doi: 10.1006/viro.1994.1120. [DOI] [PubMed] [Google Scholar]
- Zanoni R. G., Nauta I. M., Pauli U., Peterhans E. Expression in Escherichia coli and sequencing of the coding region for the capsid protein of Dutch maedi-visna virus strain ZZV 1050: application of recombinant protein in enzyme-linked immunosorbent assay for the detection of caprine and ovine lentiviruses. J Clin Microbiol. 1991 Jul;29(7):1290–1294. doi: 10.1128/jcm.29.7.1290-1294.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni R., Krieg A., Peterhans E. Detection of antibodies to caprine arthritis-encephalitis virus by protein G enzyme-linked immunosorbent assay and immunoblotting. J Clin Microbiol. 1989 Mar;27(3):580–582. doi: 10.1128/jcm.27.3.580-582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M. C., Narayan O., Kennedy P. G., Clements J. E. Pathogenesis of visna/maedi and caprine arthritis-encephalitis: new leads on the mechanism of restricted virus replication and persistent inflammation. Vet Immunol Immunopathol. 1987 May;15(1-2):167–180. doi: 10.1016/0165-2427(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Zink M. C., Yager J. A., Myers J. D. Pathogenesis of caprine arthritis encephalitis virus. Cellular localization of viral transcripts in tissues of infected goats. Am J Pathol. 1990 Apr;136(4):843–854. [PMC free article] [PubMed] [Google Scholar]
- van de Putte L. B., Lens J. W., van den Berg W. B., Kruijsen M. W. Exacerbation of antigen-induced arthritis after challenge with intravenous antigen. Immunology. 1983 May;49(1):161–167. [PMC free article] [PubMed] [Google Scholar]