Abstract
Cardiovascular gene therapy is a novel approach to the treatment of diseases such as congestive heart failure (CHF). Gene transfer to the heart would allow for the replacement of defective or missing cellular proteins that may improve cardiac performance. Our laboratory has been focusing on the feasibility of restoring β-adrenergic signaling deficiencies that are a characteristic of chronic CHF. We have now studied isolated ventricular myocytes from rabbits that have been chronically paced to produce hemodynamic failure. We document molecular β-adrenergic signaling defects including down-regulation of myocardial β-adrenergic receptors (β-ARs), functional β-AR uncoupling, and an up-regulation of the β-AR kinase (βARK1). Adenoviral-mediated gene transfer of the human β2-AR or an inhibitor of βARK1 to these failing myocytes led to the restoration of β-AR signaling. These results demonstrate that defects present in this critical myocardial signaling pathway can be corrected in vitro using genetic modification and raise the possibility of novel inotropic therapies for CHF including the inhibition of βARK1 activity in the heart.
Signaling through myocardial β-adrenergic receptors (β-ARs) plays a critical role in normal and compromised heart function. Congestive heart failure (CHF), a condition resulting from a variety of cardiovascular disorders, is a leading cause of morbidity and mortality in this country, and hospitalizations secondary to CHF are dramatically increasing (1). When the heart fails, a constellation of biochemical defects has been noted which include significant alterations in the β-AR system. Dysfunctional β-AR signaling in CHF includes receptor down-regulation and impaired signaling through remaining receptors (2, 3), possibly due to enhanced activity of the β-AR kinase (βARK1) which has been shown to be elevated in human CHF (4). βARK1 is a member of the G protein-coupled receptor kinase (GRK) family that can phosphorylate and desensitize agonist-occupied β-ARs (5). Despite an increased understanding of the molecular defects in CHF and improvements in pharmacological therapies for this clinical problem, successful long-term solutions have been elusive. β-Agonists, for example, are quite effective in acute settings, but have relatively poor efficacy chronically due, in part, to further down-regulation and desensitization of β-ARs (6, 7).
We have previously reported that myocardial-targeted overexpression of β2-ARs (8) or an inhibitor of βARK1 activity (9) in transgenic mice leads to significantly enhanced myocardial β-AR signaling and in vivo left ventricular (LV) contractility. In these transgenic mice, chronically enhanced β-AR-mediated contractility achieved by two different mechanisms (i.e., receptor up-regulation and loss of desensitization) did not lead to any significant long-term damage to the heart, suggesting that these methods of enhancing β-AR signaling are functionally different than administration of β-agonists (10). In addition to these transgenic animals, we have reported that adenoviral-mediated gene transfer and overexpression of either β2-ARs or the βARK1 inhibitor in cultured ventricular myocytes isolated from normal adult rabbits leads to a similar potentiation of β-adrenergic signaling (11). The βARK inhibitor utilized in these studies was the carboxyl-terminal 194 amino acids of βARK1 (βARKct) which competes with endogenous βARK1 for binding to the βγ-subunits of G proteins (Gβγ), an event required for βARK translocation to the plasma membrane (5, 9).
The present study was performed to determine whether ventricular myocytes isolated from a rabbit model of CHF display molecular β-AR signaling defects similar to those seen in human CHF and to determine potential functional effects of adenoviral-mediated gene transfer of either β2-ARs or the βARKct. Specifically, we attempted to rescue cellular signaling deficiencies characterized in failing cardiomyocytes. Because the β-AR system is such a powerful modulator of cardiac function, the ability to manipulate β-AR density and/or functional coupling through genetic modification is of great interest for possible novel therapeutic modalities for CHF.
EXPERIMENTAL PROCEDURES
Tachycardia-Induced CHF in Rabbits.
All procedures and protocols were approved by the Animal Care and Use Committee of Duke University. Adult male New Zealand White rabbits (2–4 kg) were obtained and housed under standard conditions and allowed to feed ad lib. The rabbits were anesthetized with a mixture of ketamine (50 mg/kg) and xylazine (2 mg/kg), intubated, and mechanically ventilated. A left thoracotomy was performed and two insulated pacing wires were attached to the LV. Following the procedure, animals were extubated, and after they were fully awake, returned to their cages. Control animals underwent thoracotomy with implantation of pacing wires but never underwent initiation of pacing. Custom pacemakers were designed that weighed 9 g and were 2 cm × 1 cm in size. The pacer was operated by a 9 V battery that was replaced every 5 days. One week after surgery, ventricular pacing at a rate of 360 beats/min was initiated. Pacing was confirmed with electrocardiogram at the beginning of the study and on a weekly basis for the duration of the 3-week pacing period.
In Vivo Hemodynamic Data.
Immediately before the initiation of rapid ventricular pacing, rabbits were premedicated with ketamine (50 mg/kg) and acepromazine (1–2 mg/kg). A small incision was made in the neck to expose the right carotid artery. A 2.5-Fr micropressure transducer (Millar Instruments, Houston) was zeroed to atmospheric pressure and passed into the right carotid artery, down into the LV to record pressures as well as heart rate. Confirmation of the position of the catheter was obtained through the use of fluoroscopy as well as the pressure waveform. Data acquisition was recorded on a PC-based system. After 3 weeks of rapid ventricular pacing, the pacer was disconnected from the animal and the same procedure was repeated to acquire postpacing hemodynamic data.
Myocyte Isolation and Culture.
Sham-operated and paced rabbits were anesthetized, treated with heparin, and then intubated as above. Hearts were excised and perfused by the Langendorff technique as described (11) with Joklik’s modified minimum medium containing hyaluronidase, collagenase, bacterial protease, and 12.5 μM CaCl2. When the heart turned soft, the ventricles were dissected free and cells were isolated as described (11). This procedure typically yielded 1–2 × 107 myocytes per rabbit heart with 50–80% in rod-shaped morphology. Myocytes were plated at a density of 1 × 105/35-mm well or 1 × 106/100 mm on tissue culture plates that were precoated with 20 μg/ml of mouse laminin for 1 hr. The myocytes were calcium-tolerant as evidenced by their quiescent state throughout the experiments.
Adenoviral Infection.
After 5 hr the myocytes adhered to the tissue culture plates and cells were infected with the appropriate titer of adenovirus in 1-ml culture medium for 100 mm plates and 0.5 ml for 6-well dishes. The cells were incubated with the virus for 15 min, after which culture medium was added back to the plates. The adenoviral vector (type 5) utilized has been described (11). Myocytes were either infected with an empty adenoviral vector (empty Ad5) or adenoviruses containing the transgenes for the human β2-AR (Adeno-β2AR) or the βARKct (Adeno-βARKct).
Membrane and Cytosolic Preparation.
Thirty-six hr after adenoviral infection, cells were harvested in lysis buffer (5 mM Tris⋅HCl, pH 7.4/5 mM EDTA) and dounce homogenized with 15 strokes on ice. Samples were centrifuged at 40,000 × g for 15 min to pellet membranes and the supernatants were concentrated in a Centricon-10 filter apparatus (Amicon). Concentrated supernatants were kept at 0°C. Membranes were resuspended in lysis buffer and stored in aliquots at −80°C.
Intracellular cAMP Assay.
Cells were labeled overnight in 3.0 μCi/ml (1 Ci = 37 GBq) [3H]adenine (DuPont/NEN) in medium 199 and then preincubated in minimal essential medium with 10 mM Hepes and 1 mM 3-isobutyl-1- methylxanthine (IBMX) for 30 min as described (11). For time course experiments, the cells were subsequently stimulated with 10 μM (−)-isoproterenol (ISO) in medium containing 100 μM ascorbic acid. For dose-response studies the ISO concentration is explicitly stated and the cells were incubated for 15 min. Following incubation, the medium was aspirated and 1 ml of ice-cold stop solution (2.5% perchloric acid/100 μM cAMP/10,000 cpm 14C) was added to each well. cAMP was determined by anion exchange chromatography, and a percent incorporation of the total 3H uptake was calculated (11).
Radioligand Binding Assays.
Binding assays were performed on 25 μg of membrane protein using saturating amounts of the β-AR-specific ligand [125I]cyanopindolol (300 pM). Nonspecific binding was determined in the presence of 20 μM alprenolol. Reactions were conducted in 500 μl of binding buffer (75 mM Tris⋅HCl, pH 7.4/12.5 mM MgCl2/2 mM EDTA) at 37°C for 1 hr and terminated by vacuum-filtration through glass-fiber filters. All assays were performed in triplicate, and receptor density was normalized to mg of membrane protein (11).
GRK Activity Assays.
Membrane extracts were prepared as described (9, 12). Concentrated membrane extracts (100 μg of protein) were incubated with rhodopsin-enriched rod outer segment membranes in 75 μl of GRK lysis buffer (25 mM Tris⋅HCl, pH 7.4/5 mM EDTA/5 mM EGTA/10 μg of leupeptin/ml/20 μg of aprotinin/ml/1 mM phenyl-methylsulfonyl fluoride) with 10 mM MgCl2 and 0.1 mM ATP containing [γ-32P]ATP. The reactions were incubated in white light for 15 min at room temperature and quenched with 300 μl of ice-cold lysis buffer and then centrifuged for 15 min at 13,000 × g. Sedimented proteins were resuspended in 25 μl of protein–gel loading dye and electrophoresed through SDS/12% polyacrylamide gels. Phosphorylated rhodopsin was visualized by autoradiography of dried gels and GRK-mediated phosphate incorporation was quantified using a PhosphorImager (Molecular Dynamics).
Sarcolemmal Membrane Adenylyl Cyclase Activity.
Crude myocardial membranes were prepared as described above from both control and failing hearts. Membranes (20–30 μg of protein) were incubated for 15 min at 37°C with [α-32P]ATP under basal conditions or in the presence of either progressive doses of ISO or 10 mM NaF and cAMP was quantitated by standard methods (8).
Protein Immunoblotting.
Whole-cell extracts were prepared by dounce homogenization in 5 mM Tris⋅HCl, pH 7.4/5 mM EDTA. A total of 80 μg of protein for each control and CHF ventricular myocyte extract sample was electrophoresed through 12% Tris/glycine gels and transferred to nitrocellulose. Membranes were blocked in 5% nonfat dried milk in 0.1% Tween 20 in PBS (PBS-T) for 1 hr at room temperature. The blots were then washed in PBS-T for 15 min and then incubated with a monoclonal βARK antibody (13) diluted 1:10,000 in 10 ml of PBS-T for 1 hr at room temperature. βARK1 was visualized using an anti-mouse/horseradish peroxidase-linked secondary antibody and ECL detection (Amersham). For protein immunoblotting of Gαs and Gαi, membrane fractions were prepared as described above, and protein immunoblots were carried out on 20 μg of membrane protein using commercially available antibodies raised against Gαs or Gαi1–3 (Santa Cruz Biotechnology). For immunoblotting of the βARKct after gene transfer, cytosolic extracts were prepared as described (11), and the 30-kDa peptide was visualized after incubation with polyclonal βARK antiserum raised against this peptide (9, 11).
Statistical Analysis.
Data are expressed as mean ± SEM. Hemodynamic values comparing pre- and postpacing measurements and signaling data from different groups of myocytes were evaluated using a Student’s t test. An ANOVA with repeated measurements plus a grouping factor was performed on the ISO dose-response data from various myocyte groups. For all analyses, P < 0.05 was considered significant.
RESULTS
Physiological Effects of Chronic Tachycardia.
Rapid ventricular pacing in rabbits has been used to successfully produce a model of CHF (14, 15). In this study, none of the animals died during surgical instrumentation, although there was a 10% mortality rate over the course of the 3-week pacing period. Twenty-one days of chronic ventricular pacing at a rate of 360 beats/min resulted in gross clinical evidence of CHF in rabbits including biventricular dilatation, pleural effusions, and abdominal ascites. In vivo hemodynamic measurements also demonstrated LV physiologic failure (Table 1). Heart rate and LV pressures were recorded in each rabbit prior to surgical implantation of the pacemaker and again after the pacing period (with pacemaker turned off). Thus, each rabbit served as its own control. Control sham-operated rabbits showed no difference in hemodynamics at the end of the 3-week study period.
Table 1.
Hemodynamic measurements after 3 weeks of ventricular pacing
| Control | CHF | P value | |
|---|---|---|---|
| HR, bpm | 187 ± 3 | 199 ± 4 | 0.07 |
| LVSP, mm Hg | 69 ± 2 | 60 ± 2 | 0.01 |
| LVEDP, mm Hg | 2.5 ± 0.4 | 7.8 ± 0.4 | 0.00003 |
| LV + dP/dtmax, mm Hg/sec | 1,916 ± 67 | 1,472 ± 37 | 0.0003 |
| LV − dP/dtmin, mm Hg/sec | 1,521 ± 73 | 1,416 ± 60 | 0.29 |
For Control (prepacing values) and CHF (postpacing values measured with pacemaker off), n = 6. HR, heart rate [beats/min (bpm)]; LVSP, LV systolic pressure; LVEDP, LV end-diastolic pressure; LV + dP/dtmax, maximum rate of LV pressure development; LV − dP/dtmin, maximum rate of LV pressure decline. P values, Student’s t test.
β-Adrenergic Signaling Abnormalities.
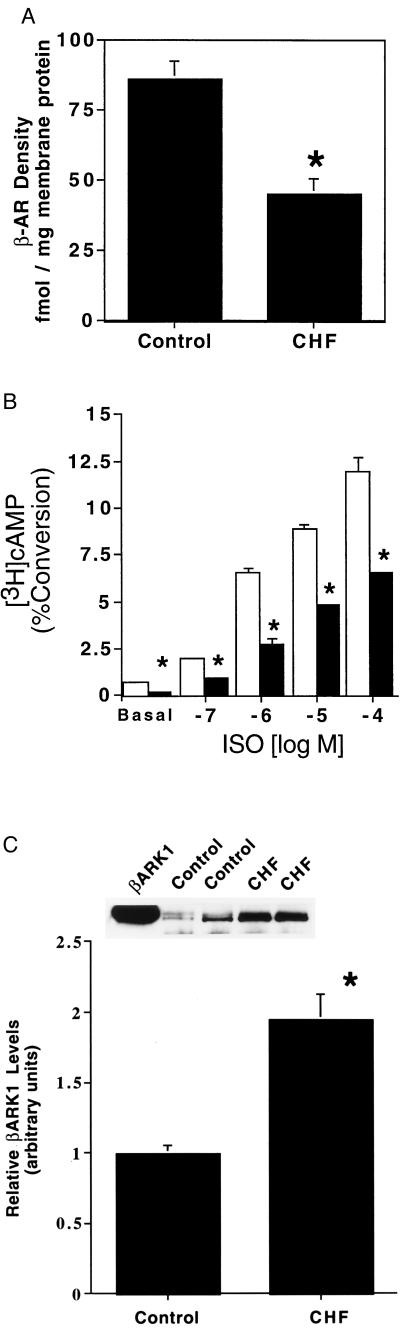
To assess β-AR signaling defects in the hearts of paced rabbits, ventricular myocytes were isolated and a series of molecular analyses were done. β-AR density in sarcolemmal membranes was determined and as shown in Fig. 1A, total β-AR density was significantly diminished in failing myocytes compared with sham-operated control myocytes approaching a 50% decrease. This is similar to the level of β-AR down-regulation seen in chronic human CHF (2). To assess functional coupling of myocardial β-ARs, we studied adenylyl cyclase activity in intact ventricular myocytes by measuring intracellular cAMP accumulation. Basal cAMP production was depressed in failing myocytes (0.23 ± 0.03% conversion of [3H]adenine to [3H]cAMP, n = 5) compared with control (sham) myocytes (0.60 ± 0.16%, n = 5, P < 0.05) (Fig. 1B). In addition, there was significant attenuation of adenylyl cyclase activity in failing myocytes in response to progressive stimulation with ISO compared with the responses of control myocytes (Fig. 1B).
Figure 1.
β-AR signaling defects in ventricular myocytes isolated from chronically paced rabbit hearts. (A) β-AR density was determined from myocardial sarcolemmal membranes prepared from ventricular myocytes isolated from control (sham-operated) or CHF (paced) rabbits (n = 5 in each group; ∗, P < 0.05 vs. control). (B) Basal and ISO-stimulated intracellular adenylyl cyclase activity was determined for control (open bars) and CHF ventricular myocytes (filled bars) (n = 5 myocyte preparations each; ∗ P < 0.05 vs. control). (C) Levels of expression of βARK1 in whole-cell extracts were assessed by protein immunoblotting. Shown is the average data for five myocyte preparations isolated from control (sham) and CHF (paced) rabbit hearts. βARK1 densitometry values from control immunoblots were arbitrarily set to 1 and all values were normalized to controls. The Inset shows a representative protein immunoblot from two control and two CHF preparations with purified βARK1 used as a marker (∗, P < 0.05 vs. control).
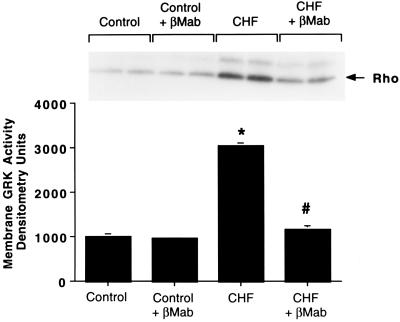
Because the cellular cAMP responses were depressed in failing cardiomyocytes, we examined the expression of βARK1 which has been shown to be elevated in human CHF (4). To assess the expression of βARK1, we performed protein immunoblotting with a βARK mAb (13, 16). In whole-cell extracts prepared from failing and control myocytes, there was a significant ≈2-fold increase in βARK1 protein levels in the CHF cells (Fig. 1C). To further assess the significance and functional consequences of βARK1 up-regulation, we studied GRK activity in the membrane fraction of ventricular myocytes using an in vitro rhodopsin phosphorylation assay. Although βARK1 is primarily a cytosolic enzyme, it requires a membrane translocation event before its activation. This membrane-localized receptor-targeted event is mediated by specific binding of the carboxyl terminus of βARK1 and Gβγ (4, 10). As shown in Fig. 2, membrane extracts from failing myocytes had ≈3-fold higher GRK activity than extracts from control myocytes. To directly determine whether this significant increase in GRK activity is due to enhanced membrane βARK1 activity or another GRK such as the membrane-associated GRK5 (12), the assay was repeated in the presence of the βARK1 mAb. It has been shown previously that this antibody selectively inhibits βARK1 activity and does not affect GRK5 activity (13, 16). As shown in Fig. 2, the βARK1 antibody virtually abolished the increased GRK activity in the CHF myocyte membrane extracts whereas control myocyte membrane GRK activity was unaltered by addition of the βARK1 antibody. When an antibody specific for GRK5 was added to the assay in addition to the βARK1 antibody, both failing and control myocyte membrane GRK activity was abolished (data not shown). Thus, basal GRK activity in control myocyte membranes is primarily due to GRK5, whereas CHF results in significant increases primarily in βARK1 expression and membrane translocation. However, some increase in the membrane GRK activity in CHF may be due to GRK5.
Figure 2.
Myocyte membrane GRK activity. GRK activity was determined by an in vitro rhodopsin phosphorylation assay for control (sham) and CHF (paced) ventricular myocyte membrane extracts in the absence and presence of a βARK mAb (βMab) which selectively inhibits βARK activity. Shown is the mean of five separate myocyte preparations with the inset depicting a representative experiment done in duplicate. Phosphorylated rhodopsin is marked by the arrow (∗, P < 0.05 vs. control; #, P < 0.05 vs. CHF).
As a final step in our biochemical analysis of failing cardiomyocytes, we assessed protein levels of the α-subunits of the G proteins Gs and Gi that regulate myocardial adenylyl cyclase activity. In human CHF, the expression and activity of the cyclase inhibitory Gαi has been shown to be elevated (17). Protein immunoblotting revealed that membrane extracts from CHF myocytes had control levels of the cyclase stimulatory Gαs, but a 2-fold increase in Gαi (data not shown). Thus, myocytes isolated from paced hearts display biochemical alterations in the β-AR-G protein-adenylyl cyclase pathway identical to what has been found in human CHF.
Expression of Adenoviral Transgenes.
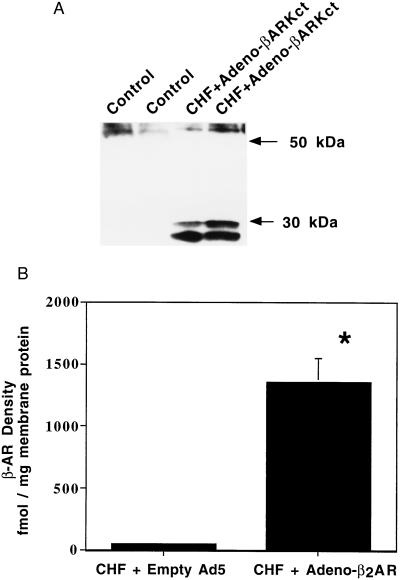
Prior to determining the functional consequences of adenovirus infection in failing cardiomyocytes, we examined the expression of the adenoviral transgenes. The transgenes utilized were the human β2-AR (Adeno-β2AR) and the βARKct (Adeno-βARKct) acting as a βARK1 inhibitor (9, 11). Control myocytes were infected with an empty adenoviral vector (empty Ad5). Myocytes were infected with adenoviruses at a multiplicity of infection (moi) of 100:1, which we have previously determined to produce optimal transgene expression in 100% of rabbit ventricular myocytes (11). Thirty-six hours after Adeno-βARKct infection, extracts from CHF myocytes expressed significant levels of the ≈30-kDa βARK1 inhibitor peptide as determined by protein immunoblots (Fig. 3A). β2-AR transgene expression was assessed in membranes purified from Adeno-β2AR infected CHF myocytes by radioligand binding. Failing myocytes infected with the empty Ad5 had an average β-AR density of 48.5 ± 5.3 fmol/mg membrane protein, which did not differ from uninfected failing myocytes (n = 4 each). Following Adeno-β2AR infection, failing myocytes had a 30-fold increase in β-AR density (1,353.8 ± 194.5 fmol/mg membrane protein, n = 5) which is ≈15-fold over control values (Fig. 3B).
Figure 3.
Expression of the adenoviral transgenes in failing ventricular myocytes. (A) Representative Western blot displaying expression of the βARKct peptide in only Adeno-βARKct infected myocytes. (B) Total β-AR density determined for CHF ventricular myocyte membranes infected with Empty Ad5 (n = 4) or Adeno-β2AR (n = 5) (∗, P < 0.005 vs. CHF + Ad5).
Functional Effects of β2-AR and βARKct Gene Transfer in Failing Myocytes.
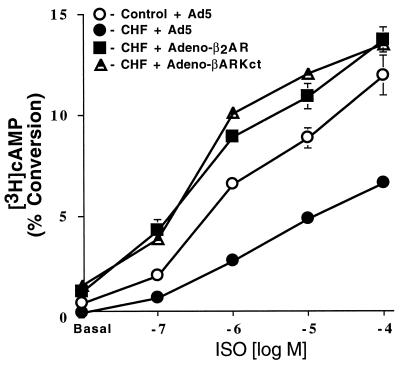
To determine the functional effects of these two β-adrenergic based transgenes on defective β-AR-mediated signaling in failing cardiomyocytes, intracellular cAMP production and accumulation was studied following adenoviral-mediated gene transfer. As shown in Fig. 4, infection of failing myocytes with Adeno-βARKct significantly increased basal intracellular cAMP accumulation in failing myocytes compared with equivalent myocytes infected with empty adenovirus (1.9 ± 0.6% vs. 0.8 ± 0.12%, n = 5 in each group, P < 0.05). This enhancement of signaling was also seen after ISO stimulation (Fig. 4). Ventricular myocytes isolated from paced rabbits and infected with Adeno-β2AR also had an increase in basal and ISO-stimulated intracellular cAMP production when compared with failing myocytes treated with the empty adenoviral vector (Fig. 4). This is significant because we found increased levels of Gαi in the failing myocytes, demonstrating that any effects of increased Gi are overcome by enhanced β-AR signaling. Interestingly, the level of basal and agonist-stimulated β-adrenergic signaling seen in failing myocytes after infection with Adeno-βARKct or Adeno-β2AR was not only significantly elevated compared with empty Ad5-infected CHF myocytes, but significantly increased to levels seen in normal ventricular myocytes (Fig. 4).
Figure 4.
Intracellular cAMP production in failing ventricular myocytes following adenoviral-mediated gene transfer. After 36 hr of infection with the appropriate adenovirus, control (sham) or CHF myocytes (n = 4 preparations each) were stimulated with increasing concentrations of ISO. Accumulation of cAMP was expressed as percent incorporation of the total [3H] uptake into [3H]cAMP. Responses for CHF cells infected with Adeno-β2AR and Adeno-βARKct were significantly elevated compared with both CHF cells (P < 0.005, ANOVA) and control cells (P < 0.005, ANOVA) infected with Ad5.
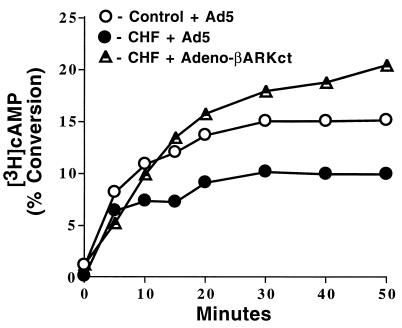
To determine whether myocardial β-AR desensitization in failing myocytes was inhibited by the expression of the βARKct, the time course of agonist-stimulated intracellular cAMP production was studied. This data is shown in Fig. 5. Enhanced β-AR desensitization is apparent in CHF myocytes as there is insignificant cAMP accumulation after 5 min compared with control myocytes, which show linear accumulation up to 10 min before there is a decline in the rate due to desensitization mechanisms. In contrast, CHF myocytes infected with the βARKct show linear cAMP accumulation well after 10 min and have not plateaued even at 50 min after ISO administration.
Figure 5.
Time course of cAMP production. Assessment of the effect of Adeno-βARKct infection on β-AR desensitization via a time course of cAMP production in response to 10 μM ISO. Myocytes were isolated from control (sham) rabbit hearts or CHF (paced) rabbit hearts and infected with Empty Ad5 or Adeno-βARKct (n = 4 each) and cAMP was measured over the course of 50 min.
DISCUSSION
In this study, we report the novel finding that β-adrenergic signaling defects, present in failing ventricular cardiomyocytes, can be corrected via adenoviral-mediated gene transfer. Deficient β-AR signaling was reversed by either overexpression of receptor molecules or inhibition of βARK1-mediated β-AR desensitization using a peptide inhibitor of this GRK (βARKct). The adenoviral-mediated overexpression of the βARKct and significant improvement in the signaling of failing cardiomyocytes provides strong evidence for the importance of βARK1 in the pathogenesis of CHF and provides a novel therapeutic target for the production of positive inotropy. Our first goal in the present study was to characterize any β-AR alterations present in myocytes isolated from chronically paced rabbits which were in hemodynamic failure (Table 1). Molecular analysis of the β-AR system in these myocytes indicates that this rabbit model of CHF shares biochemical properties seen in human CHF. We found significant down-regulation of β-ARs and functional uncoupling of the adenylyl cyclase response as assessed by β-AR-mediated intracellular cAMP accumulation (Fig. 1B). We also observed that the negative regulatory proteins βARK1 (Figs. 1C and 3) and Gαi were elevated. These molecular changes likely contribute to the cardiac dysfunction seen in CHF, particularly the loss of β-AR inotropic reserve. Although previous studies using this experimental rabbit model of CHF demonstrated functional deficiencies including those present in isolated cardiomyocytes (14, 15), none have addressed specific molecular changes concerning the β-AR signaling system as reported here.
The discovery that βARK1 protein expression and activity are elevated in this model of CHF (as in human CHF) adds to recent reports that this GRK is altered during several other cardiovascular disorders such as myocardial ischemia in the rat heart (18), pressure overload hypertrophy in the mouse (16), and mild human hypertension (19). Thus, it is becoming increasingly apparent that βARK1, and GRK activity in general, is critically involved in the pathophysiology of CHF as well as other cardiovascular disorders. In the present study, the increased GRK activity was seen in membrane fractions where GRK5 is also expressed (12), however, antibody inhibition studies demonstrated that the enhanced GRK activity was primarily due to βARK1 (Fig. 2).
Our laboratory has been studying the physiological consequences of altering the expression of myocardial proteins including adrenergic receptors and GRKs. This was first done using transgenic mouse models where cardiac-specific overexpression of β2-ARs (8) or the βARKct (9) dramatically increased cardiac contractility. Conversely, myocardial-targeted overexpression in transgenic mice of βARK1 (9) or GRK5 (12) significantly depressed cardiac function. In addition, we have also reported the effects of the β2-AR and βARKct transgenes on the β-adrenergic signaling properties of normal rabbit ventricular myocytes isolated in culture (11). Adenoviral-mediated gene transfer of these two transgenes produced effects on β-AR-mediated myocardial adenylyl cyclase activity which were similar to those observed in the transgenic mice, indicating that alterations in β-AR signaling are possible following acute gene transfer.
Taken together, these studies in transgenic mice and normal cardiomyocytes led to our hypothesis that increasing β-AR signaling may improve the functional performance of the failing heart. Myocytes isolated from rabbit hearts with tachycardia-induced CHF provide a powerful opportunity to test this hypothesis. Infection of failing ventricular myocytes with Adeno-β2AR and Adeno-βARKct leads to dramatic overexpression of these proteins (Fig. 3). A 30-fold overexpression of the β2-AR led to significant increases in cAMP production both basally and after β-agonist stimulation (Fig. 4) indicating that these overexpressed receptors are functionally coupled.
Perhaps the most significant result from this study is the finding that cellular inhibition of βARK1 by overexpression of the βARKct peptide not only reverses the β-AR-mediated signaling defects of failing cardiomyocytes but improves functional coupling of endogenous β-ARs to normal levels (Fig. 4). Desensitization was clearly attenuated by Adeno-βARKct infection as the cellular cAMP response to ISO did not plateau during the course of the experiment while failing myocytes infected with an empty adenovirus did not show significant cAMP accumulation after 5 min (Fig. 5), consistent with cAMP responses in myocytes isolated from normal control rabbits (11). In the case of the failing myocyte, the action of βARKct effectively inhibits the 3-fold excess of βARK1 activity seen in these cells. Therefore, the βARKct cannot only inhibit the endogenous activity of βARK1 in myocytes (9, 11, 20) but also the increased activity that occurs in CHF. In addition, we have recently demonstrated that βARKct expression in hypertrophied hearts also reverses β-AR-mediated cardiac dysfunction due to increased βARK1 expression and activity (16). Because it is becoming increasingly apparent that βARK1 is involved in the pathophysiology of several cardiovascular disorders, inhibition of βARK1-mediated β-AR desensitization may have therapeutic implications beyond the effects seen here in failing cardiomyocytes. Further substantiating the potential importance of βARK1 activity as a therapeutic target is the finding that chronic administration of a β-antagonist to pigs resulted in lower myocardial GRK activity (21). Interestingly, β-antagonists have also been shown to improve mortality in patients with chronic CHF (22).
In conclusion, this study has demonstrated that impaired β-AR-mediated signaling in ventricular myocytes, including enhanced Gαi expression, can be rescued with adenoviral-mediated gene transfer by either restoring β-AR density or inhibiting βARK1 activity. Thus, this study, in the setting of CHF, is consistent with our original findings in transgenic mice that β2-ARs and βARK1 are critical regulators of myocardial function and represent novel potential targets for the future development of gene therapy strategies to enhance cardiac performance in CHF. In the case of βARK1, pharmaceutical inhibitors of this critical GRK could also be developed which might have similar effects. This would eliminate the need for gene transfer protocols which are still years away from practical applications in chronic CHF.
Acknowledgments
We thank Drs. M. Drazner, S. Dyer, and R. E. Lilly for help with preliminary experiments. We also thank Ms. K. Wilson for technical assistance and M. Shiflett for excellent secretarial support. This study was supported in part by National Institutes of Health Grants HL-16037 (R.J.L.) and National Research Service Award HL-09436 (S.A.A).
ABBREVIATIONS
- CHF
congestive heart failure
- β-AR
β-adrenergic receptor
- βARK1
β-AR kinase
- GRK
G protein-coupled receptor kinase
- LV
left ventricular
- βARKct
carboxyl-terminal 194 amino acids of βARK1
- ISO
(−)-isoproterenol
References
- 1.Williams R S. N Engl J Med. 1995;332:817–818. doi: 10.1056/NEJM199503233321212. [DOI] [PubMed] [Google Scholar]
- 2.Bristow M R, Ginsburg R, Minobe W, Cubicciotti R, Sageman W S, Lurie K, Billingham M E, Harrison D C, Stinson E B. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 3.Brodde O E, Michel M C, Zerkowski H R. Cardiovasc Res. 1995;30:570–584. [PubMed] [Google Scholar]
- 4.Ungerer M, Bohm M, Elce J S, Erdmann E, Lohse M L. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 5.Inglese J, Freedman N J, Koch W J, Lefkowitz R J. J Biol Chem. 1993;268:23735–23738. [PubMed] [Google Scholar]
- 6.Bristow M R, Lowes B D. Coronary Artery Dis. 1994;5:112–118. doi: 10.1097/00019501-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Xamoterol in Heart Failure Study Group. Lancet. 1990;336:1–6. [PubMed] [Google Scholar]
- 8.Milano C A, Allen L F, Rockman H A, Dolber P C, McMinn T R, Chien K R, Johnson T D, Bond R A, Lefkowitz R J. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 9.Koch W J, Rockman H A, Samama P, Hamilton R A, Bond R A, Milano C A, Lefkowitz R J. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 10.Koch W J, Milano C A, Lefkowitz R J. Circ Res. 1996;78:511–516. doi: 10.1161/01.res.78.4.511. [DOI] [PubMed] [Google Scholar]
- 11.Drazner M H, Peppel K C, Dyer S, Grant A O, Koch W J, Lefkowitz R J. J Clin Invest. 1997;99:288–296. doi: 10.1172/JCI119157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockman H A, Choi D-J, Rahman N U, Akhter S A, Lefkowitz R J, Koch W J. Proc Natl Acad Sci USA. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opperman M, Diverse-Pierluisse M, Drazner M H, Dyer S L, Freedman N J, Peppel K C, Lefkowitz R J. Proc Natl Acad Sci USA. 1996;93:7649–7654. doi: 10.1073/pnas.93.15.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masaki H, Imaizumi T, Ando S, Hirooka Y, Harada S, Momohara M, Nagano M, Takeshita A. Cardiovasc Res. 1993;27:828–831. doi: 10.1093/cvr/27.5.828. [DOI] [PubMed] [Google Scholar]
- 15.Spinale F G, Eble D M, Mukherjee R, Johnson W S, Walker J D. Basic Res Cardiol. 1994;89:456–467. doi: 10.1007/BF00788282. [DOI] [PubMed] [Google Scholar]
- 16.Choi D-J, Koch W J, Hunter J J, Rockman H A. J Biol Chem. 1997;272:17223–17229. doi: 10.1074/jbc.272.27.17223. [DOI] [PubMed] [Google Scholar]
- 17.Feldman A M, Cates A E, Veazey W B, Hershberger R E, Bristow M R, Baughman K L, Baumgartner W A, Van Dop C. J Clin Invest. 1988;82:189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungerer M, Kessebohm K, Kronsbein K, Lohse M J, Richardt G. Circ Res. 1996;79:455–460. doi: 10.1161/01.res.79.3.455. [DOI] [PubMed] [Google Scholar]
- 19.Gros R, Benovic J L, Tan C M, Feldman R D. J Clin Invest. 1997;99:2087–2093. doi: 10.1172/JCI119381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korzick D H, Xiao R P, Ziman B D, Koch W J, Lefkowitz R J, Lakatta E G. Am J Physiol. 1997;272:H590–H596. doi: 10.1152/ajpheart.1997.272.1.H590. [DOI] [PubMed] [Google Scholar]
- 21.Ping P, Gelzer-Bell R, Roth D A, Kiel D, Insel P A, Hammond H K. J Clin Invest. 1995;95:1271–1280. doi: 10.1172/JCI117777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Packer M, Bristow M R, Cohn J N, Colucci W S, Fowler M B, Gilbert E M, Shusterman H. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]