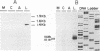Abstract
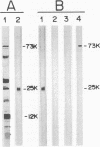
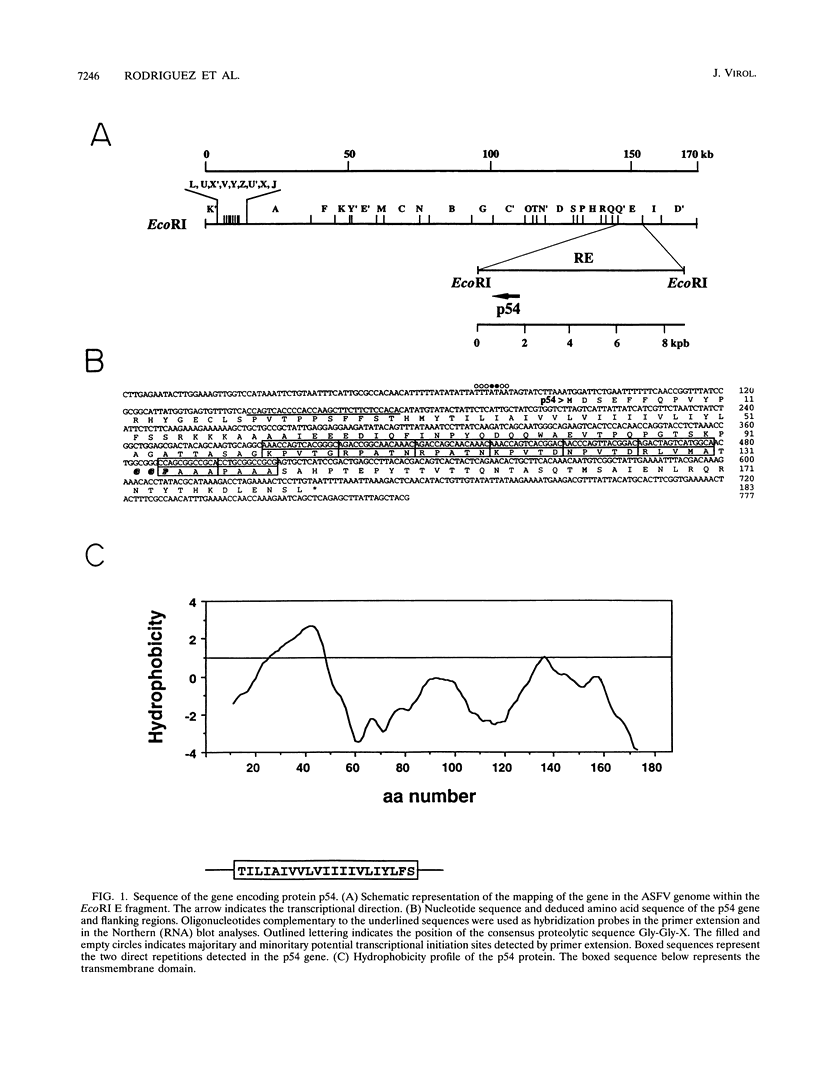
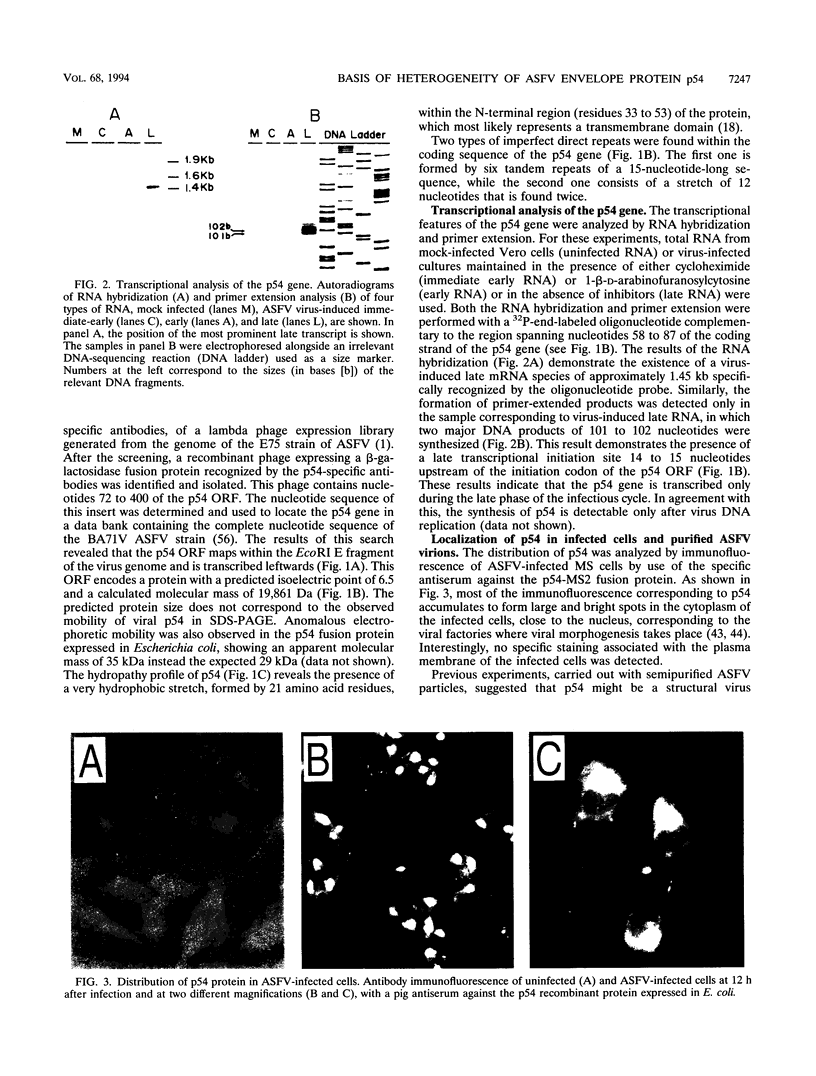
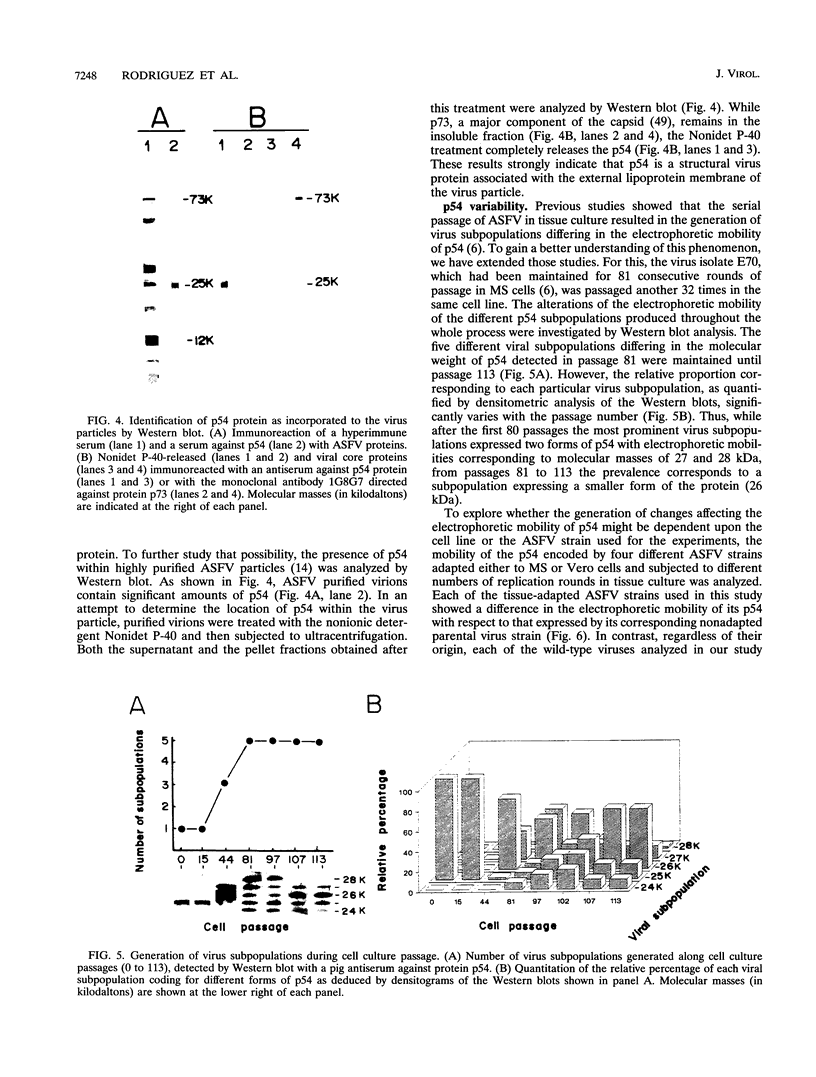
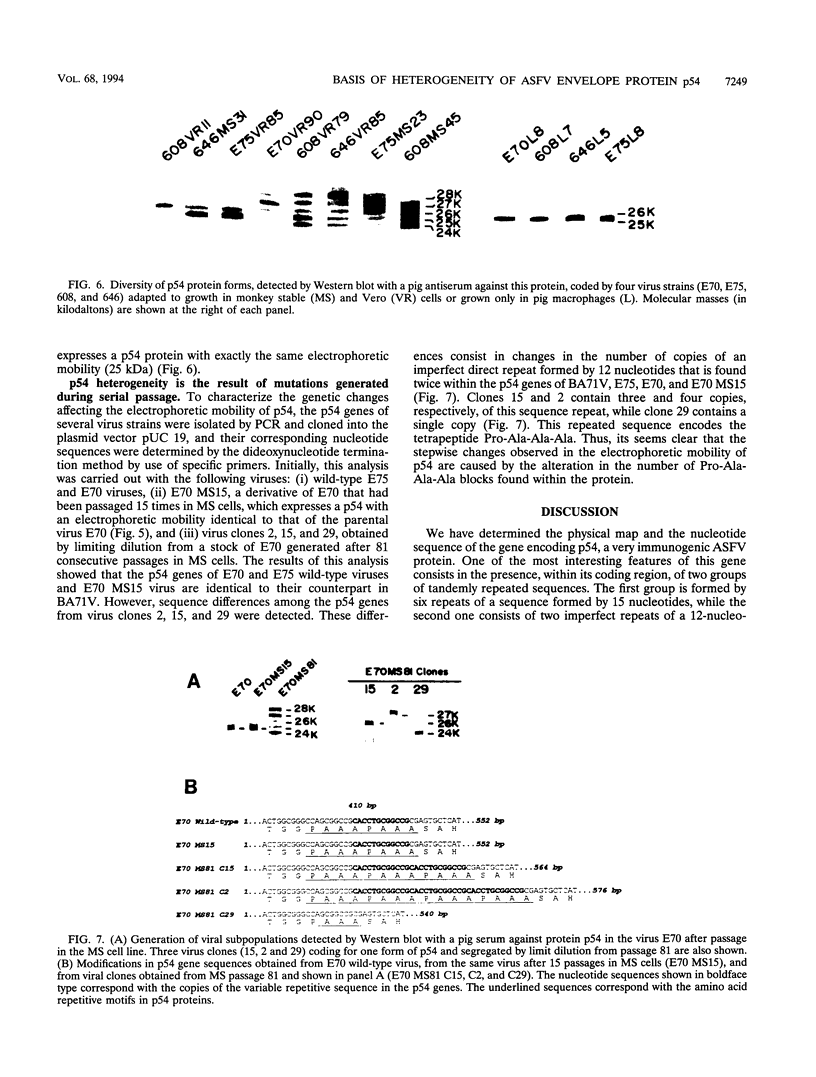
It has been reported that the propagation of African swine fever virus (ASFV) in cell culture generates viral subpopulations differing in protein p54 (C. Alcaraz, A. Brun, F. Ruiz-Gonzalvo, and J. M. Escribano, Virus Res. 23:173-182, 1992). A recombinant bacteriophage expressing a 328-bp fragment of the p54 gene was selected in a lambda phage expression library of ASFV genomic fragments by immunoscreening with antibodies against p54 protein. The sequence of this recombinant phage allowed the location of the p54 gene in the EcoRI E fragment of the ASFV genome. Nucleotide sequence obtained from this fragment revealed an open reading frame encoding a protein of 183 amino acids with a calculated molecular weight of 19,861. This protein contains a transmembrane domain and a Gly-Gly-X motif, a recognition sequence for protein processing of several ASFV structural proteins. In addition, two direct tandem repetitions were also found within this open reading frame. Further characterization of the transcription and gene product revealed that the p54 gene is translated from a late mRNA and the protein is incorporated to the external membrane of the virus particle. A comparison of the nucleotide sequence of the p54 gene carried by two virulent ASFV strains (E70 and E75) with that obtained from virus Ba71V showed 100% similarity. However, when p54 genes from viral clones generated by cell culture passage and coding for p54 proteins with different electrophoretic mobility were sequenced, they showed changes in the number of copies of a 12-nucleotide sequence repeat. These changes produce alterations in the number of copies of the amino acid sequence Pro-Ala-Ala-Ala present in p54, resulting in stepwise modifications in the molecular weight of the protein. These duplications and deletions of a tandem repeat sequence array within a protein coding region constitute a novel mechanism of genetic diversification in ASFV.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afonso C. L., Alcaraz C., Brun A., Sussman M. D., Onisk D. V., Escribano J. M., Rock D. L. Characterization of p30, a highly antigenic membrane and secreted protein of African swine fever virus. Virology. 1992 Jul;189(1):368–373. doi: 10.1016/0042-6822(92)90718-5. [DOI] [PubMed] [Google Scholar]
- Aguado B., Selmes I. P., Smith G. L. Nucleotide sequence of 21.8 kbp of variola major virus strain Harvey and comparison with vaccinia virus. J Gen Virol. 1992 Nov;73(Pt 11):2887–2902. doi: 10.1099/0022-1317-73-11-2887. [DOI] [PubMed] [Google Scholar]
- Agüero M., Blasco R., Wilkinson P., Viñuela E. Analysis of naturally occurring deletion variants of African swine fever virus: multigene family 110 is not essential for infectivity or virulence in pigs. Virology. 1990 May;176(1):195–204. doi: 10.1016/0042-6822(90)90244-l. [DOI] [PubMed] [Google Scholar]
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Alcaraz C., Alvarez A., Escribano J. M. Flow cytometric analysis of African swine fever virus-induced plasma membrane proteins and their humoral immune response in infected pigs. Virology. 1992 Jul;189(1):266–273. doi: 10.1016/0042-6822(92)90702-q. [DOI] [PubMed] [Google Scholar]
- Alcaraz C., Brun A., Ruiz-Gonzalvo F., Escribano J. M. Cell culture propagation modifies the African swine fever virus replication phenotype in macrophages and generates viral subpopulations differing in protein p54. Virus Res. 1992 Apr;23(1-2):173–182. doi: 10.1016/0168-1702(92)90076-l. [DOI] [PubMed] [Google Scholar]
- Alcaraz C., De Diego M., Pastor M. J., Escribano J. M. Comparison of a radioimmunoprecipitation assay to immunoblotting and ELISA for detection of antibody to African swine fever virus. J Vet Diagn Invest. 1990 Jul;2(3):191–196. doi: 10.1177/104063879000200307. [DOI] [PubMed] [Google Scholar]
- Almazán F., Rodríguez J. M., Andrés G., Pérez R., Viñuela E., Rodriguez J. F. Transcriptional analysis of multigene family 110 of African swine fever virus. J Virol. 1992 Nov;66(11):6655–6667. doi: 10.1128/jvi.66.11.6655-6667.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazán F., Rodríguez J. M., Angulo A., Viñuela E., Rodriguez J. F. Transcriptional mapping of a late gene coding for the p12 attachment protein of African swine fever virus. J Virol. 1993 Jan;67(1):553–556. doi: 10.1128/jvi.67.1.553-556.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo A., Viñuela E., Alcamí A. Comparison of the sequence of the gene encoding African swine fever virus attachment protein p12 from field virus isolates and viruses passaged in tissue culture. J Virol. 1992 Jun;66(6):3869–3872. doi: 10.1128/jvi.66.6.3869-3872.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A. High-frequency homologous recombination in vaccinia virus DNA. J Virol. 1987 Jun;61(6):1788–1795. doi: 10.1128/jvi.61.6.1788-1795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco R., Agüero M., Almendral J. M., Viñuela E. Variable and constant regions in African swine fever virus DNA. Virology. 1989 Feb;168(2):330–338. doi: 10.1016/0042-6822(89)90273-0. [DOI] [PubMed] [Google Scholar]
- Blasco R., de la Vega I., Almazán F., Agüero M., Viñuela E. Genetic variation of African swine fever virus: variable regions near the ends of the viral DNA. Virology. 1989 Nov;173(1):251–257. doi: 10.1016/0042-6822(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Carrascosa A. L., del Val M., Santarén J. F., Viñuela E. Purification and properties of African swine fever virus. J Virol. 1985 May;54(2):337–344. doi: 10.1128/jvi.54.2.337-344.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collick A., Jeffreys A. J. Detection of a novel minisatellite-specific DNA-binding protein. Nucleic Acids Res. 1990 Feb 11;18(3):625–629. doi: 10.1093/nar/18.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L. K., Bristow C., Wilkinson P. J., Sumption K. J. Identification of a variable region of the African swine fever virus genome that has undergone separate DNA rearrangements leading to expansion of minisatellite-like sequences. J Mol Biol. 1990 Dec 5;216(3):677–688. doi: 10.1016/0022-2836(90)90391-X. [DOI] [PubMed] [Google Scholar]
- Escribano J. M., Pastor M. J., Sánchez-Vizcaíno J. M. Antibodies to bovine serum albumin in swine sera: implications for false-positive reactions in the serodiagnosis of African swine fever. Am J Vet Res. 1989 Jul;50(7):1118–1122. [PubMed] [Google Scholar]
- García-Barreno B., Sanz A., Nogal M. L., Viñuela E., Enjuanes L. Monoclonal antibodies of African swine fever virus: antigenic differences among field virus isolates and viruses passaged in cell culture. J Virol. 1986 May;58(2):385–392. doi: 10.1128/jvi.58.2.385-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess W. R. African swine fever: a reassessment. Adv Vet Sci Comp Med. 1981;25:39–69. [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- López-Otín C., Simón-Mateo C., Martínez L., Viñuela E. Gly-Gly-X, a novel consensus sequence for the proteolytic processing of viral and cellular proteins. J Biol Chem. 1989 Jun 5;264(16):9107–9110. [PubMed] [Google Scholar]
- MALMQUIST W. A., HAY D. Hemadsorption and cytopathic effect produced by African Swine Fever virus in swine bone marrow and buffy coat cultures. Am J Vet Res. 1960 Jan;21:104–108. [PubMed] [Google Scholar]
- McGeoch D. J. The genomes of the human herpesviruses: contents, relationships, and evolution. Annu Rev Microbiol. 1989;43:235–265. doi: 10.1146/annurev.mi.43.100189.001315. [DOI] [PubMed] [Google Scholar]
- Nunes J. F., Vigário J. D., Terrinha A. M. Ultrastructural study of African swine fever virus replication in cultures of swine bone marrow cells. Arch Virol. 1975;49(1):59–66. doi: 10.1007/BF02175596. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Pan I. C., Hess W. R. Diversity of African swine fever virus. Am J Vet Res. 1985 Feb;46(2):314–320. [PubMed] [Google Scholar]
- Pastor M. J., Arias M., Escribano J. M. Comparison of two antigens for use in an enzyme-linked immunosorbent assay to detect African swine fever antibody. Am J Vet Res. 1990 Oct;51(10):1540–1543. [PubMed] [Google Scholar]
- Plowright W., Parker J., Peirce M. A. African swine fever virus in ticks (Ornithodoros moubata, murray) collected from animal burrows in Tanzania. Nature. 1969 Mar 15;221(5185):1071–1073. doi: 10.1038/2211071a0. [DOI] [PubMed] [Google Scholar]
- Prieto J., Candel E., Coloma A. Nucleotide sequence of a cDNA encoding ribosomal acidic phosphoprotein P1 from Dictyostelium discoideum: identification of a novel carboxy-terminal sequence in 'A' proteins. Nucleic Acids Res. 1991 Mar 25;19(6):1340–1340. doi: 10.1093/nar/19.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. M., Salas M. L., Viñuela E. Genes homologous to ubiquitin-conjugating proteins and eukaryotic transcription factor SII in African swine fever virus. Virology. 1992 Jan;186(1):40–52. doi: 10.1016/0042-6822(92)90059-x. [DOI] [PubMed] [Google Scholar]
- Rodríguez J. M., Yáez R. J., Almazán F., Viñuela E., Rodriguez J. F. African swine fever virus encodes a CD2 homolog responsible for the adhesion of erythrocytes to infected cells. J Virol. 1993 Sep;67(9):5312–5320. doi: 10.1128/jvi.67.9.5312-5320.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs D. H., Leight G., Cone J., Schwarz S., Stuart L., Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976 Dec;22(6):559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarén J. F., Viñuela E. African swine fever virus-induced polypeptides in Vero cells. Virus Res. 1986 Sep;5(4):391–405. doi: 10.1016/0168-1702(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Santurde G., Ruiz Gonzalvo F., Carnero M. E., Tabarés E. Genetic stability of African swine fever virus grown in monkey kidney cells. Brief report. Arch Virol. 1988;98(1-2):117–122. doi: 10.1007/BF01321012. [DOI] [PubMed] [Google Scholar]
- Sanz A., García-Barreno B., Nogal M. L., Viñuela E., Enjuanes L. Monoclonal antibodies specific for African swine fever virus proteins. J Virol. 1985 Apr;54(1):199–206. doi: 10.1128/jvi.54.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Mateo C., Andrés G., Viñuela E. Polyprotein processing in African swine fever virus: a novel gene expression strategy for a DNA virus. EMBO J. 1993 Jul;12(7):2977–2987. doi: 10.1002/j.1460-2075.1993.tb05960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Howard S. T. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J Gen Virol. 1991 Jun;72(Pt 6):1349–1376. doi: 10.1099/0022-1317-72-6-1349. [DOI] [PubMed] [Google Scholar]
- Strebel K., Beck E., Strohmaier K., Schaller H. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J Virol. 1986 Mar;57(3):983–991. doi: 10.1128/jvi.57.3.983-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumption K. J., Hutchings G. H., Wilkinson P. J., Dixon L. K. Variable regions on the genome of Malawi isolates of African swine fever virus. J Gen Virol. 1990 Oct;71(Pt 10):2331–2340. doi: 10.1099/0022-1317-71-10-2331. [DOI] [PubMed] [Google Scholar]
- Tabarés E., Marcotegui M. A., Fernández M., Sánchez-Botija C. Proteins specified by African swine fever virus. I. Analysis of viral structural proteins and antigenic properties. Arch Virol. 1980;66(2):107–117. doi: 10.1007/BF01314979. [DOI] [PubMed] [Google Scholar]
- Tabarés E., Olivares I., Santurde G., Garcia M. J., Martin E., Carnero M. E. African swine fever virus DNA: deletions and additions during adaptation to growth in monkey kidney cells. Arch Virol. 1987;97(3-4):333–346. doi: 10.1007/BF01314431. [DOI] [PubMed] [Google Scholar]
- Vazquez M. P., Schijman A. G., Levin M. J. Nucleotide sequence of a cDNA encoding a Trypanosoma cruzi acidic ribosomal P1 type protein. Nucleic Acids Res. 1992 May 25;20(10):2599–2599. doi: 10.1093/nar/20.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez M. P., Schijman A. G., Panebra A., Levin M. J. Nucleotide sequence of a cDNA encoding another Trypanosoma cruzi acidic ribosomal P2 type protein (TcP2b). Nucleic Acids Res. 1992 Jun 11;20(11):2893–2893. doi: 10.1093/nar/20.11.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D., Pan I. C. African swine fever virus DNA: restriction endonuclease cleavage patterns of wild-type, Vero cell-adapted and plaque-purified virus. J Gen Virol. 1982 Dec;63(2):383–391. doi: 10.1099/0022-1317-63-2-383. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega I., Viñuela E., Blasco R. Genetic variation and multigene families in African swine fever virus. Virology. 1990 Nov;179(1):234–246. doi: 10.1016/0042-6822(90)90293-z. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]