Abstract
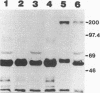
Aminopeptidase-N (APN) has been identified [B. Delmas, J. Gelfi, R. L'Haridon, L. K. Vogel, H. Sjostrom, O. Noren, and H. Laude, Nature (London) 357:417-420, 1992] as a major receptor for porcine transmissible gastroenteritis virus (TGEV). Binding of TGEV to villous enterocytes from the jejuna of newborn pigs is saturable and at a higher level than that of binding of virus to newborn cryptal enterocytes or to enterocytes from older piglets (H. M. Weingartl and J. B. Derbyshire, Vet. Microbiol. 35:23-32, 1993). The distribution of APN in enterocytes in the jejuna of neonatal and 3 week-old-piglets, as determined by the measurement of enzymatic activity and by labeling of the cells with an anti-APN monoclonal antibody, did not correspond with the reported distribution of saturable binding sites on enterocytes. Monoclonal antibodies, which were prepared against plasma membranes derived from enterocytes harvested from the upper villi of newborn pigs, blocked the replication of TGEV, but not the porcine respiratory coronavirus, in ST cells and immunoprecipitated a 200-kDa protein in ST cell lysates. This protein was demonstrated by immunohistochemistry and by fluorescence-activated cell scanning to be present on the villous enterocytes of newborn pigs but to be lacking on the cryptal enterocytes of newborn pigs and on the villous and cryptal enterocytes of 3-week-old piglets. Since this distribution of the protein corresponds to the previously demonstrated distribution of saturable binding sites, we conclude that the 200-kDa protein may be an additional receptor for TGEV which is restricted to the villous enterocytes of newborn pigs and which contributes to the age sensitivity of these animals to the virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cepica A., Derbyshire J. B. Antibody-dependent and spontaneous cell-mediated cytotoxicity against transmissible gastroenteritis virus infected cells by lymphocytes from sows, fetuses and neonatal piglets. Can J Comp Med. 1984 Jul;48(3):258–261. [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L. K., Sjöström H., Norén O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992 Jun 4;357(6377):417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratecos D., Knibiehler M., Benoit V., Sémériva M. Plasma membranes from rat intestinal epithelial cells at different stages of maturation. I. Preparation and characterization of plasma membrane subfractions originating from crypt cells and from villous cells. Biochim Biophys Acta. 1978 Oct 4;512(3):508–524. doi: 10.1016/0005-2736(78)90161-x. [DOI] [PubMed] [Google Scholar]
- Hanham C. A., Zhao F., Tignor G. H. Evidence from the anti-idiotypic network that the acetylcholine receptor is a rabies virus receptor. J Virol. 1993 Jan;67(1):530–542. doi: 10.1128/jvi.67.1.530-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan L. T., Derbyshire J. B. Antiviral activity of interferon against transmissible gastroenteritis virus in cell culture and ligated intestinal segments in neonatal pigs. Vet Microbiol. 1994 Jan;38(3):263–276. doi: 10.1016/0378-1135(94)90007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moon H. W. Epithelial cell migration in the alimentary mucosa of the suckling pig. Proc Soc Exp Biol Med. 1971 May;137(1):151–154. doi: 10.3181/00379727-137-35533. [DOI] [PubMed] [Google Scholar]
- Nédellec P., Dveksler G. S., Daniels E., Turbide C., Chow B., Basile A. A., Holmes K. V., Beauchemin N. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J Virol. 1994 Jul;68(7):4525–4537. doi: 10.1128/jvi.68.7.4525-4537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J., Sjöström H., Norén O. Cloning of the pig aminopeptidase N gene. Identification of possible regulatory elements and the exon distribution in relation to the membrane-spanning region. FEBS Lett. 1989 Jul 17;251(1-2):275–281. doi: 10.1016/0014-5793(89)81470-x. [DOI] [PubMed] [Google Scholar]
- Pensaert M., Haelterman E. O., Burnstein T. Transmissible gastroenteritis of swine: virus-intestinal cell interactions. I. Immunofluorescence, histopathology and virus production in the small intestine through the course of infection. Arch Gesamte Virusforsch. 1970;31(3):321–334. doi: 10.1007/BF01253767. [DOI] [PubMed] [Google Scholar]
- Pensaert M., Haelterman E. O., Hinsman E. J. Transmissible gastroenteritis of swine: virus-intestinal cell interactions. II. Electron microscopy of the epithelium in isolated jejunal loops. Arch Gesamte Virusforsch. 1970;31(3):335–351. doi: 10.1007/BF01253768. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Allen J. C., Harigaya S. Possible involvement of cardiac Na+, K+-adenosine triphosphatase in the mechanism of action of cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):31–41. [PubMed] [Google Scholar]
- Sears A. E., McGwire B. S., Roizman B. Infection of polarized MDCK cells with herpes simplex virus 1: two asymmetrically distributed cell receptors interact with different viral proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5087–5091. doi: 10.1073/pnas.88.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepley M. P., Racaniello V. R. A monoclonal antibody that blocks poliovirus attachment recognizes the lymphocyte homing receptor CD44. J Virol. 1994 Mar;68(3):1301–1308. doi: 10.1128/jvi.68.3.1301-1308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Jeppesen L., Staun M., Svensson B., Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. Eur J Biochem. 1978 Aug 1;88(2):503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Jarvis L. G. Growth and cell replacement in the new-born pig intestine. Proc R Soc Lond B Biol Sci. 1978 Nov 20;203(1150):69–89. doi: 10.1098/rspb.1978.0092. [DOI] [PubMed] [Google Scholar]
- Sánchez C. M., Gebauer F., Suñ C., Mendez A., Dopazo J., Enjuanes L. Genetic evolution and tropism of transmissible gastroenteritis coronaviruses. Virology. 1992 Sep;190(1):92–105. doi: 10.1016/0042-6822(92)91195-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. E., Beamer P. D., Ristic M. Electron microscopy of intestinal epithelial cells of piglets infected with a transmissible gastroenteritis virus. Can J Comp Med. 1973 Apr;37(2):177–188. [PMC free article] [PubMed] [Google Scholar]
- Weingartl H. M., Derbyshire J. B. Binding of porcine transmissible gastroenteritis virus by enterocytes from newborn and weaned piglets. Vet Microbiol. 1993 May;35(1-2):23–32. doi: 10.1016/0378-1135(93)90113-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Welch S. K., Saif L. J. Monoclonal antibodies to a virulent strain of transmissible gastroenteritis virus: comparison of reactivity with virulent and attenuated virus. Arch Virol. 1988;101(3-4):221–235. doi: 10.1007/BF01311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W., Minocha H. C. Identification of the cell surface receptor for bovine viral diarrhoea virus by using anti-idiotypic antibodies. J Gen Virol. 1993 Jan;74(Pt 1):73–79. doi: 10.1099/0022-1317-74-1-73. [DOI] [PubMed] [Google Scholar]
- Yeager C. L., Ashmun R. A., Williams R. K., Cardellichio C. B., Shapiro L. H., Look A. T., Holmes K. V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992 Jun 4;357(6377):420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]