Abstract
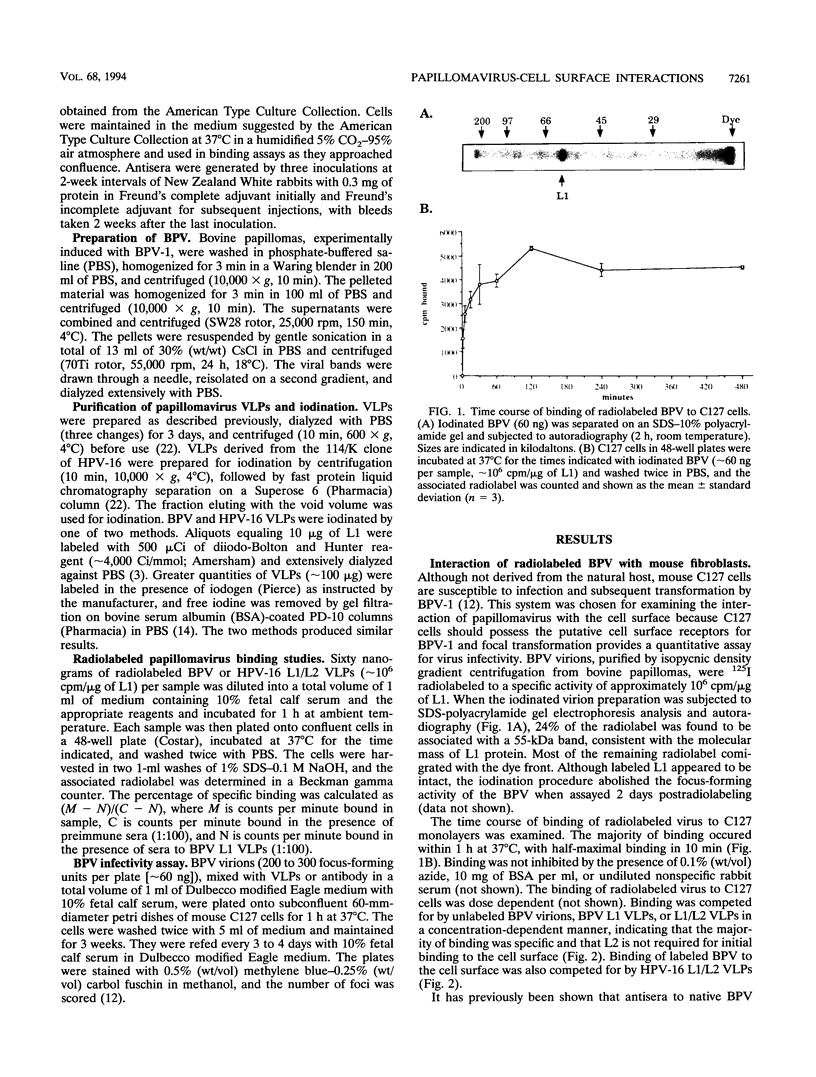
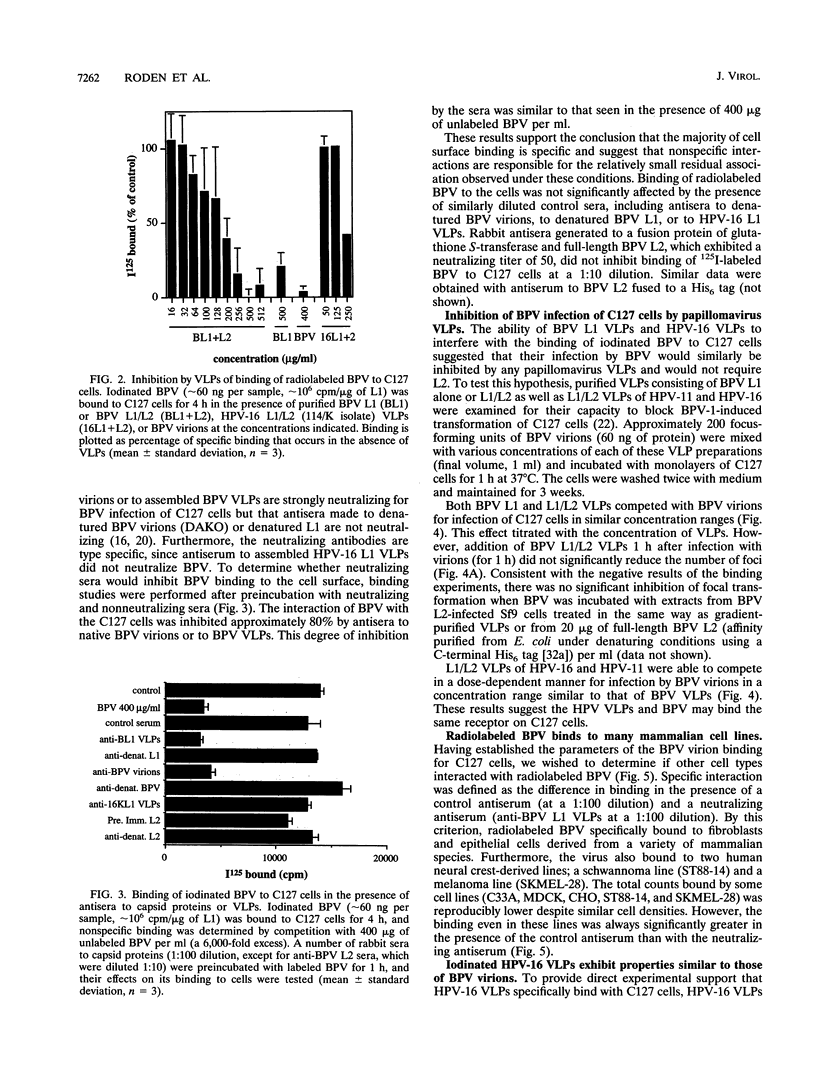
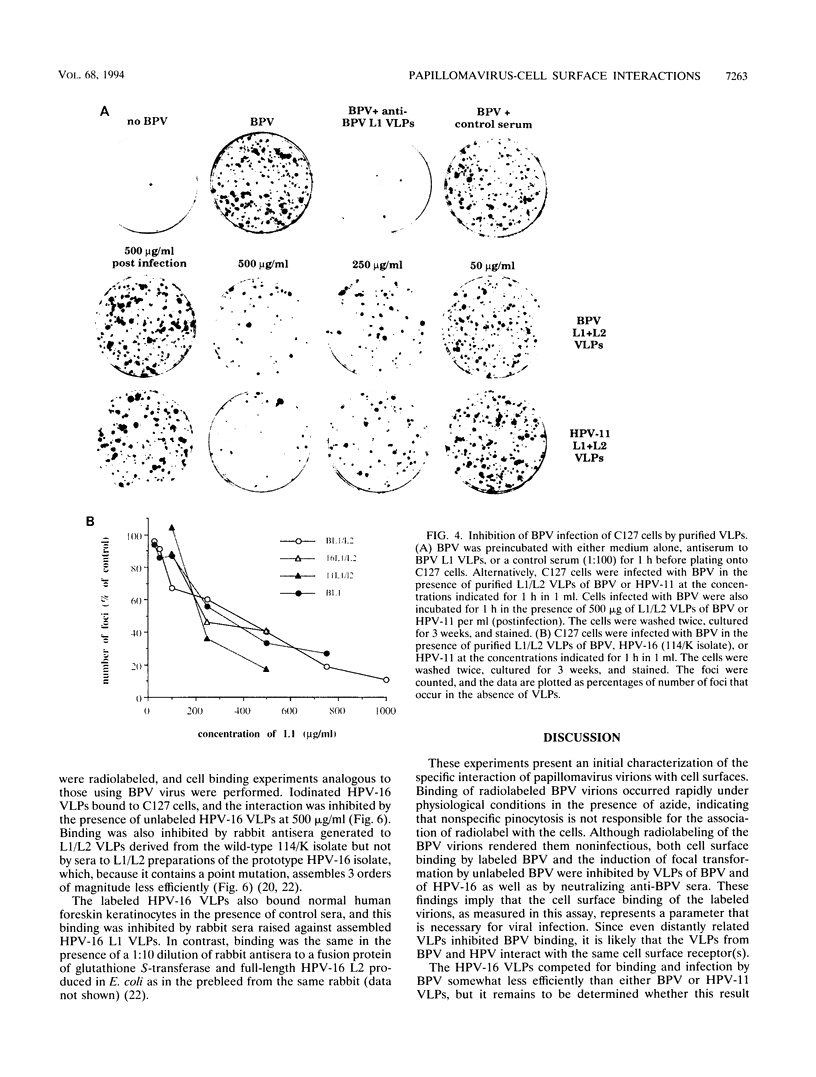
To initiate an investigation of the initial step in papillomavirus infection, we have examined the interaction of bovine papillomavirus type 1 (BPV) virions with C127 cells by two assays, binding of radioiodinated BPV virions to cell monolayers and BPV-induced focal transformation. Under physiological conditions, the labeled virions bound to the cell surface in a dose-dependent manner within 1 h. Antibody studies indicated that the interaction was specific and related to infectivity: polyclonal sera raised to BPV virions or to baculovirus-expressed BPV L1 virus-like particles (VLPs) inhibited BPV binding and focal transformation, while sera to denatured BPV virions, to denatured BPV L1, or to human papillomavirus type 16 (HPV-16) VLPs were not inhibitory. An exception was that antisera to BPV L2 were neutralizing but did not inhibit binding. Unlabeled BPV virions and BPV VLPs competed with binding to the cell surface in a concentration-dependent manner. Binding to the cell surface appeared to depend primarily on L1, since BPV VLPs composed of L1 alone or of L1/L2 were equally effective in inhibiting binding and focal transformation. VLPs of HPV-16 also inhibited BPV binding and BPV transformation of C127 cells, suggesting that they interact with the same cell surface molecule(s) as BPV virions. Radiolabeled BPV bound specifically to several mammalian cell lines of fibroblastic and epithelial origin, as well as to a human schwannoma and melanoma lines, although some lines bound up to 10 times as many counts as others. Radiolabeled HPV-16 VLPs bound to both human keratinocytes and mouse C127 cells. The results suggest that papillomaviruses bind a widely expressed and evolutionarily conserved cell surface receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. S., Newcomb W. W., Olson N. H., Cowsert L. M., Olson C., Brown J. C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991 Dec;60(6):1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Vass W. C., Lowy D. R., Schiller J. T. In vitro biological activities of the E6 and E7 genes vary among human papillomaviruses of different oncogenic potential. J Virol. 1991 Jan;65(1):292–298. doi: 10.1128/jvi.65.1.292-298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason J., Khan S. A., Best J. M. Towards vaccines against human papillomavirus type-16 genital infections. Vaccine. 1993;11(6):603–611. doi: 10.1016/0264-410x(93)90302-e. [DOI] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J Virol. 1990 Jul;64(7):3151–3156. doi: 10.1128/jvi.64.7.3151-3156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W., Cladel N. M., Patrick S. D., Welsh P. A. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol. 1990 Nov;64(11):5678–5681. doi: 10.1128/jvi.64.11.5678-5681.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W., Kan N. C., DiAngelo S. L. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991 Apr;181(2):572–579. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W. Monoclonal antibody neutralization of BPV-1. Virus Res. 1993 May;28(2):195–202. doi: 10.1016/0168-1702(93)90136-b. [DOI] [PubMed] [Google Scholar]
- Compton T., Nowlin D. M., Cooper N. R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993 Apr;193(2):834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- Crawford L. Prospects for cervical cancer vaccines. Cancer Surv. 1993;16:215–229. [PubMed] [Google Scholar]
- Dollard S. C., Wilson J. L., Demeter L. M., Bonnez W., Reichman R. C., Broker T. R., Chow L. T. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. OFF. Genes Dev. 1992 Jul;6(7):1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Fletcher J. A., Kozakewich H. P., Hoffer F. A., Lage J. M., Weidner N., Tepper R., Pinkus G. S., Morton C. C., Corson J. M. Diagnostic relevance of clonal cytogenetic aberrations in malignant soft-tissue tumors. N Engl J Med. 1991 Feb 14;324(7):436–442. doi: 10.1056/NEJM199102143240702. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fried H., Cahan L. D., Paulson J. C. Polyoma virus recognizes specific sialyligosaccharide receptors on host cells. Virology. 1981 Feb;109(1):188–192. doi: 10.1016/0042-6822(81)90485-2. [DOI] [PubMed] [Google Scholar]
- Ghim S., Christensen N. D., Kreider J. W., Jenson A. B. Comparison of neutralization of BPV-1 infection of C127 cells and bovine fetal skin xenografts. Int J Cancer. 1991 Sep 9;49(2):285–289. doi: 10.1002/ijc.2910490224. [DOI] [PubMed] [Google Scholar]
- Hagensee M. E., Yaegashi N., Galloway D. A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993 Jan;67(1):315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994 Jan;68(1):1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasawa A., Kumamoto Y., Fujinaga K. Detection of human papillomavirus deoxyribonucleic acid in penile carcinoma by polymerase chain reaction and in situ hybridization. J Urol. 1993 Jan;149(1):59–63. doi: 10.1016/s0022-5347(17)35999-2. [DOI] [PubMed] [Google Scholar]
- Kirnbauer R., Booy F., Cheng N., Lowy D. R., Schiller J. T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R., Hubbert N. L., Wheeler C. M., Becker T. M., Lowy D. R., Schiller J. T. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst. 1994 Apr 6;86(7):494–499. doi: 10.1093/jnci/86.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R., Taub J., Greenstone H., Roden R., Dürst M., Gissmann L., Lowy D. R., Schiller J. T. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993 Dec;67(12):6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller L. D., Olson C. Attempted transmission of warts from man, cattle, and horses and of deer fibroma, to selected hosts. J Invest Dermatol. 1972 Jun;58(6):366–368. doi: 10.1111/1523-1747.ep12540579. [DOI] [PubMed] [Google Scholar]
- Koutsky L. A., Holmes K. K., Critchlow C. W., Stevens C. E., Paavonen J., Beckmann A. M., DeRouen T. A., Galloway D. A., Vernon D., Kiviat N. B. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992 Oct 29;327(18):1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Leure-Dupree A. E., Zaino R. J., Weber J. A. Laboratory production in vivo of infectious human papillomavirus type 11. J Virol. 1987 Feb;61(2):590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D., Meinke W. Persistence of viral DNA in human cell cultures infected with human papilloma virus. Nature. 1975 Jul 31;256(5516):434–436. doi: 10.1038/256434a0. [DOI] [PubMed] [Google Scholar]
- Larsen P. M., Storgaard L., Fey S. J. Proteins present in bovine papillomavirus particles. J Virol. 1987 Nov;61(11):3596–3601. doi: 10.1128/jvi.61.11.3596-3601.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Borenstein L. A., Selvakumar R., Ahmed R., Wettstein F. O. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992 Apr;187(2):612–619. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- Meyers C., Frattini M. G., Hudson J. B., Laimins L. A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992 Aug 14;257(5072):971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- Robl M. G., Gordon D. E., Lee K. P., Olson C. Intracranial fibroblastic neoplasms in the hamster from bovine papilloma virus. Cancer Res. 1972 Oct;32(10):2221–2225. [PubMed] [Google Scholar]
- Rose R. C., Bonnez W., Reichman R. C., Garcea R. L. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993 Apr;67(4):1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGRE D., OLSON C., Jr, HOERLEIN A. B. Neutralization of bovine papilloma virus with serums from cattle and horses with experimental papillomas. Am J Vet Res. 1955 Oct;16(61 Pt 1):517–520. [PubMed] [Google Scholar]
- Schiffman M. H., Bauer H. M., Hoover R. N., Glass A. G., Cadell D. M., Rush B. B., Scott D. R., Sherman M. E., Kurman R. J., Wacholder S. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993 Jun 16;85(12):958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- Shieh M. T., WuDunn D., Montgomery R. I., Esko J. D., Spear P. G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992 Mar;116(5):1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. H., Foster C., Hitchcock M. E., Isseroff R. In vitro HPV-11 infection of human foreskin. J Invest Dermatol. 1993 Sep;101(3):292–295. doi: 10.1111/1523-1747.ep12365409. [DOI] [PubMed] [Google Scholar]
- Steinberg B. M., Auborn K. J., Brandsma J. L., Taichman L. B. Tissue site-specific enhancer function of the upstream regulatory region of human papillomavirus type 11 in cultured keratinocytes. J Virol. 1989 Feb;63(2):957–960. doi: 10.1128/jvi.63.2.957-960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M., Littman D. R. Viral receptors of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):725–728. doi: 10.1016/0092-8674(89)90674-0. [DOI] [PubMed] [Google Scholar]
- Zhou J., Stenzel D. J., Sun X. Y., Frazer I. H. Synthesis and assembly of infectious bovine papillomavirus particles in vitro. J Gen Virol. 1993 Apr;74(Pt 4):763–768. doi: 10.1099/0022-1317-74-4-763. [DOI] [PubMed] [Google Scholar]






