Abstract
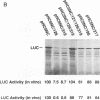
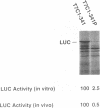
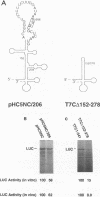
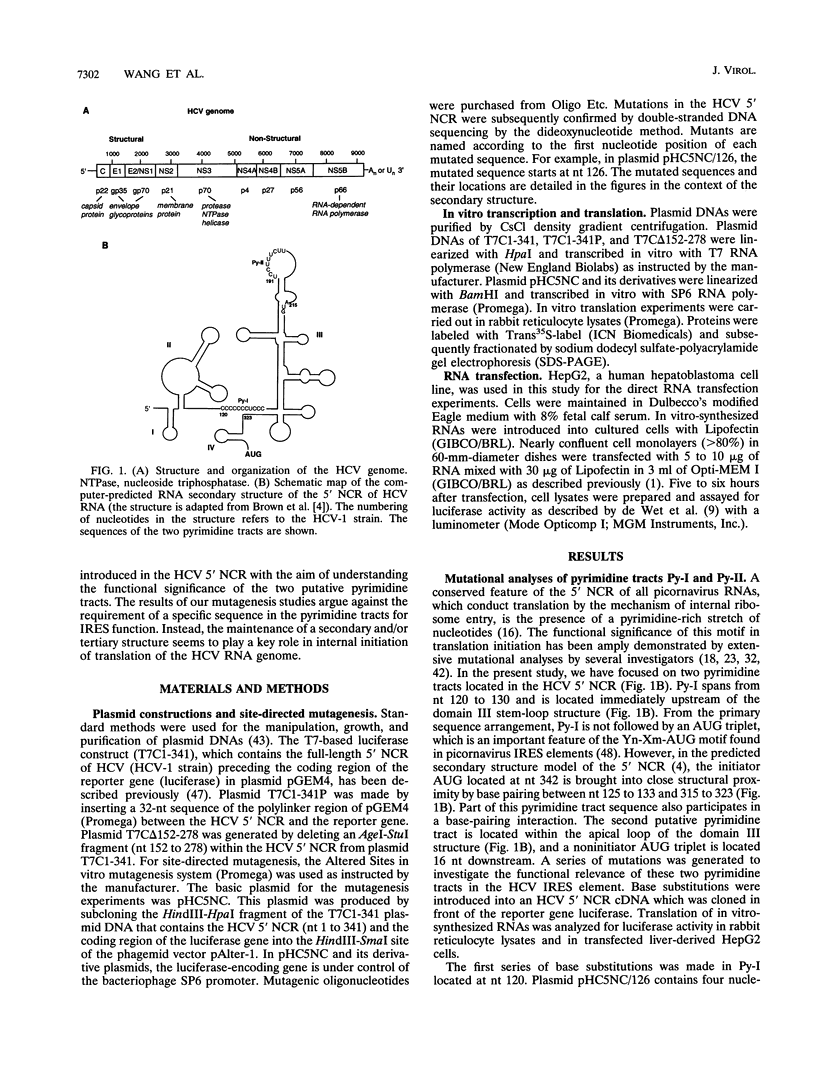
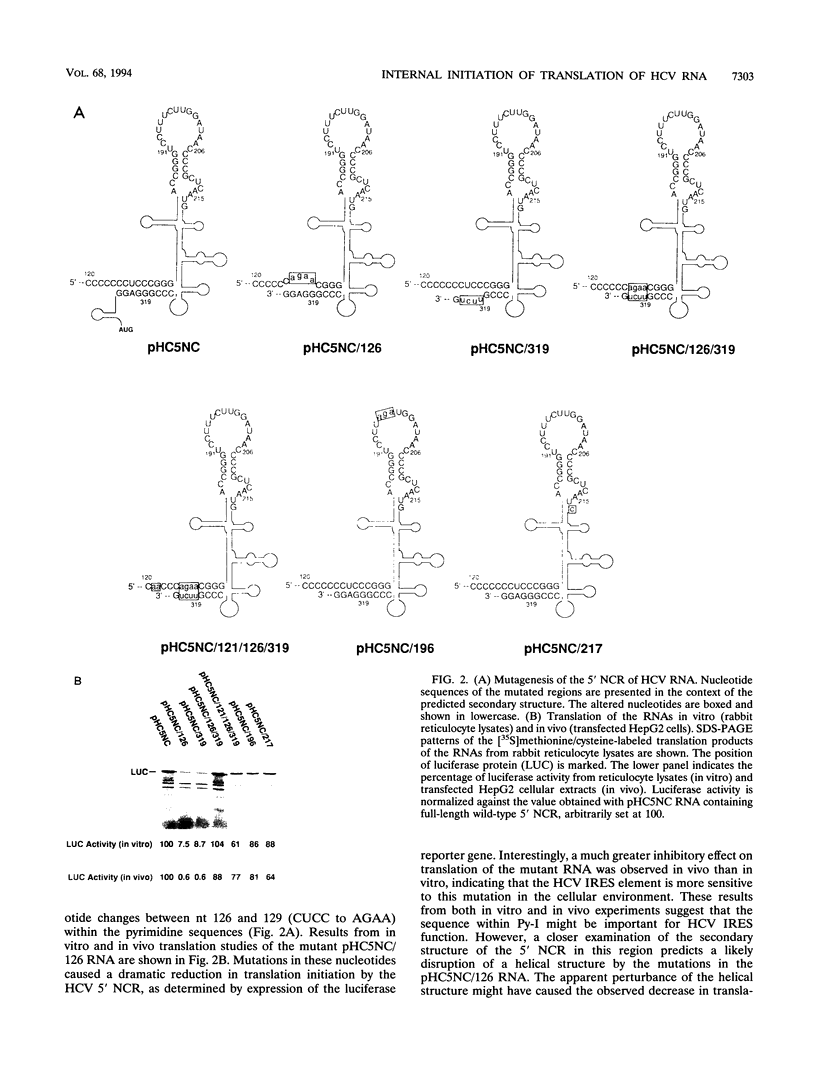
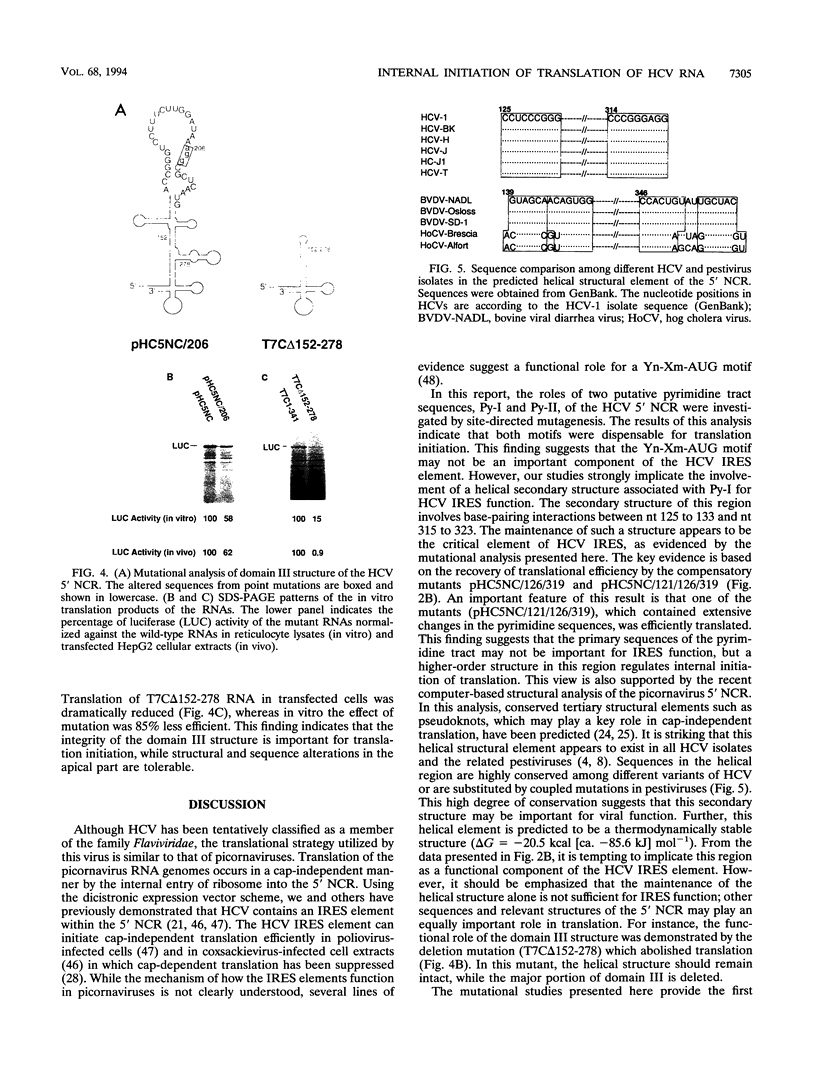
Translation of hepatitis C virus (HCV) RNA is initiated by cap-independent internal ribosome binding to the 5' noncoding region (NCR). To identify the sequences and structural elements within the 5' NCR of HCV RNA that contribute to the initiation of translation, a series of point mutations was introduced within this sequence. Since the pyrimidine-rich tract is considered a characteristic feature of picornavirus internal ribosome entry site (IRES) elements, our mutational analysis focused on two putative pyrimidine tracts (Py-I and Py-II) within the HCV 5' NCR. Translational efficiency of these mutant RNAs was examined by in vitro translation and after RNA transfection into liver-derived cells. Mutational analysis of Py-I (nucleotides 120 to 130), supported by compensatory mutants, demonstrates that the primary sequence of this motif is not important but that a helical structural element associated with this region is critical for HCV IRES function. Mutations in Py-II (nucleotides 191 to 199) show that this motif is dispensable for IRES function as well. Thus, the pyrimidine-rich tract motif, which is considered as an essential element of the picornavirus IRES elements, does not appear to be a functional component of the HCV IRES. Further, the insertional mutagenesis study suggests a requirement for proper spacing between the initiator AUG and the upstream structures of the HCV IRES element for internal initiation of translation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyopadhyay P. K., Wang C., Lipton H. L. Cap-independent translation by the 5' untranslated region of Theiler's murine encephalomyelitis virus. J Virol. 1992 Nov;66(11):6249–6256. doi: 10.1128/jvi.66.11.6249-6256.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman A., Jackson R. J. Initiation of translation of human rhinovirus RNA: mapping the internal ribosome entry site. Virology. 1992 Jun;188(2):685–696. doi: 10.1016/0042-6822(92)90523-r. [DOI] [PubMed] [Google Scholar]
- Brown E. A., Day S. P., Jansen R. W., Lemon S. M. The 5' nontranslated region of hepatitis A virus RNA: secondary structure and elements required for translation in vitro. J Virol. 1991 Nov;65(11):5828–5838. doi: 10.1128/jvi.65.11.5828-5838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. A., Zhang H., Ping L. H., Lemon S. M. Secondary structure of the 5' nontranslated regions of hepatitis C virus and pestivirus genomic RNAs. Nucleic Acids Res. 1992 Oct 11;20(19):5041–5045. doi: 10.1093/nar/20.19.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Sequence analysis of the 5' noncoding region of hepatitis C virus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4942–4946. doi: 10.1073/pnas.89.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A., Wang K. S., Overby L., Bradley D., Houghton M. Identification of the major, parenteral non-A, non-B hepatitis agent (hepatitis C virus) using a recombinant cDNA approach. Semin Liver Dis. 1992 Aug;12(3):279–288. doi: 10.1055/s-2007-1007399. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R., Brock K. V. 5' and 3' untranslated regions of pestivirus genome: primary and secondary structure analyses. Nucleic Acids Res. 1993 Apr 25;21(8):1949–1957. doi: 10.1093/nar/21.8.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildine S. L., Semler B. L. Conservation of RNA-protein interactions among picornaviruses. J Virol. 1992 Jul;66(7):4364–4376. doi: 10.1128/jvi.66.7.4364-4376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke G. M., Hoffman M. A., Palmenberg A. C. Sequence and structural elements that contribute to efficient encephalomyocarditis virus RNA translation. J Virol. 1992 Mar;66(3):1602–1609. doi: 10.1128/jvi.66.3.1602-1609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard J. R., Ehrenfeld E. Specific interactions of HeLa cell proteins with proposed translation domains of the poliovirus 5' noncoding region. J Virol. 1992 May;66(5):3101–3109. doi: 10.1128/jvi.66.5.3101-3109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M. J., Jia X. Y., Summers D. F. Identification of the hepatitis A virus internal ribosome entry site: in vivo and in vitro analysis of bicistronic RNAs containing the HAV 5' noncoding region. Virology. 1993 Apr;193(2):842–852. doi: 10.1006/viro.1993.1193. [DOI] [PubMed] [Google Scholar]
- Hellen C. U., Witherell G. W., Schmid M., Shin S. H., Pestova T. V., Gil A., Wimmer E. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M., Weiner A., Han J., Kuo G., Choo Q. L. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology. 1991 Aug;14(2):381–388. [PubMed] [Google Scholar]
- Jackson R. J., Howell M. T., Kaminski A. The novel mechanism of initiation of picornavirus RNA translation. Trends Biochem Sci. 1990 Dec;15(12):477–483. doi: 10.1016/0968-0004(90)90302-r. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990 Sep;4(9):1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Kaminski A., Belsham G. J., Jackson R. J. Translation of encephalomyocarditis virus RNA: parameters influencing the selection of the internal initiation site. EMBO J. 1994 Apr 1;13(7):1673–1681. doi: 10.1002/j.1460-2075.1994.tb06431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Luz N., Beck E. Functional analysis of the internal translation initiation site of foot-and-mouth disease virus. J Virol. 1990 Oct;64(10):4625–4631. doi: 10.1128/jvi.64.10.4625-4631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S. Y., Chen J. H., Sonenberg N., Maizel J. V. Conserved tertiary structure elements in the 5' untranslated region of human enteroviruses and rhinoviruses. Virology. 1992 Dec;191(2):858–866. doi: 10.1016/0042-6822(92)90261-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S. Y., Chen J. H., Sonenberg N., Maizel J. V., Jr Conserved tertiary structural elements in the 5' nontranslated region of cardiovirus, aphthovirus and hepatitis A virus RNAs. Nucleic Acids Res. 1993 May 25;21(10):2445–2451. doi: 10.1093/nar/21.10.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S. Y., Sonenberg N., Maizel J. V., Jr Distinct structural elements and internal entry of ribosomes in mRNA3 encoded by infectious bronchitis virus. Virology. 1994 Jan;198(1):405–411. doi: 10.1006/viro.1994.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S. Y., Zuker M. Common structures of the 5' non-coding RNA in enteroviruses and rhinoviruses. Thermodynamical stability and statistical significance. J Mol Biol. 1990 Dec 5;216(3):729–741. doi: 10.1016/0022-2836(90)90395-3. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Sonenberg N. Inactivation of cap-binding proteins accompanies the shut-off of host protein synthesis by poliovirus. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3447–3451. doi: 10.1073/pnas.79.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. X., Inglis S. C. Internal entry of ribosomes on a tricistronic mRNA encoded by infectious bronchitis virus. J Virol. 1992 Oct;66(10):6143–6154. doi: 10.1128/jvi.66.10.6143-6154.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz N., Beck E. A cellular 57 kDa protein binds to two regions of the internal translation initiation site of foot-and-mouth disease virus. FEBS Lett. 1990 Sep 3;269(2):311–314. doi: 10.1016/0014-5793(90)81182-n. [DOI] [PubMed] [Google Scholar]
- Macejak D. G., Sarnow P. Internal initiation of translation mediated by the 5' leader of a cellular mRNA. Nature. 1991 Sep 5;353(6339):90–94. doi: 10.1038/353090a0. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Nicholson R., Sonenberg N. In vitro mutational analysis of cis-acting RNA translational elements within the poliovirus type 2 5' untranslated region. J Virol. 1991 Nov;65(11):5895–5901. doi: 10.1128/jvi.65.11.5895-5901.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Pelletier J., Sonenberg N. A cellular protein that binds to the 5'-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989 Jul;3(7):1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y. V., Lee H. S., Lejbkowicz F., Kenan D. J., Chan E. K., Agol V. I., Keene J. D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993 Jul;67(7):3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R., Pelletier J., Le S. Y., Sonenberg N. Structural and functional analysis of the ribosome landing pad of poliovirus type 2: in vivo translation studies. J Virol. 1991 Nov;65(11):5886–5894. doi: 10.1128/jvi.65.11.5886-5894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. K., Scott M. P., Sarnow P. Homeotic gene Antennapedia mRNA contains 5'-noncoding sequences that confer translational initiation by internal ribosome binding. Genes Dev. 1992 Sep;6(9):1643–1653. doi: 10.1101/gad.6.9.1643. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Kurai K., Iizuka H., Machida A., Miyakawa Y., Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991 Nov;72(Pt 11):2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Percy N., Belsham G. J., Brangwyn J. K., Sullivan M., Stone D. M., Almond J. W. Intracellular modifications induced by poliovirus reduce the requirement for structural motifs in the 5' noncoding region of the genome involved in internal initiation of protein synthesis. J Virol. 1992 Mar;66(3):1695–1701. doi: 10.1128/jvi.66.3.1695-1701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Romanova L. I., Sinyakov A. N., Maslova S. V., Agol V. I. Conserved structural domains in the 5'-untranslated region of picornaviral genomes: an analysis of the segment controlling translation and neurovirulence. Virology. 1989 Feb;168(2):201–209. doi: 10.1016/0042-6822(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Pilipenko E. V., Gmyl A. P., Maslova S. V., Svitkin Y. V., Sinyakov A. N., Agol V. I. Prokaryotic-like cis elements in the cap-independent internal initiation of translation on picornavirus RNA. Cell. 1992 Jan 10;68(1):119–131. doi: 10.1016/0092-8674(92)90211-t. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Racaniello V. R., Dunn G., Cooper J., Minor P. D., Almond J. W. New model for the secondary structure of the 5' non-coding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important in neurovirulence. J Mol Biol. 1989 May 20;207(2):379–392. doi: 10.1016/0022-2836(89)90261-1. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K., Iizuka N., Kohara M., Nomoto A. Internal ribosome entry site within hepatitis C virus RNA. J Virol. 1992 Mar;66(3):1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Sarnow P., Siddiqui A. Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome-binding mechanism. J Virol. 1993 Jun;67(6):3338–3344. doi: 10.1128/jvi.67.6.3338-3344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer E., Hellen C. U., Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- Yoo B. J., Spaete R. R., Geballe A. P., Selby M., Houghton M., Han J. H. 5' end-dependent translation initiation of hepatitis C viral RNA and the presence of putative positive and negative translational control elements within the 5' untranslated region. Virology. 1992 Dec;191(2):889–899. doi: 10.1016/0042-6822(92)90264-p. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]