Abstract
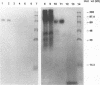
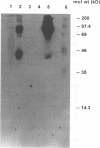
The attachment of encephalomyocarditis (EMC) virus to human nucleated cells susceptible to virus infection was examined with HeLa and K562 cell lines. Both cell types showed specific virus binding competitively blocked by unlabeled virions. The number of binding sites for EMC virus on HeLa and K562 cells were approximately 1.6 x 10(5) and 3.5 x 10(5) per cell, respectively, and dissociation binding constants were 1.1 and 2.7 nM, respectively. Treatment of cells with cycloheximide after pretreatment with trypsin eliminated EMC virus attachment, suggesting that the virus-binding moiety is proteinaceous in nature. Digestion of cells, cell membranes, and sodium deoxycholate-solubilized cell membranes with proteases or neuraminidases or treatment of cells with lectins demonstrated that the EMC virus-cell interaction is mediated by a sialoglycoprotein. Proteins with a molecular mass of 70 kDa were isolated from detergent-solubilized cell membranes of both HeLa and K562 cells by EMC virus affinity chromatography. The purified proteins, as well as their 70-kDa-molecular-mass equivalents detected in intact surface membranes of HeLa and K562 cells, specifically bound EMC virus in a virus overlay protein blot assay, whereas membranes from nonpermissive K562 D clone cells did not. Western immunoblot analysis with glycophorin A-specific antibody confirmed that the identified 70-kDa binding site on K562 cells is not glycophorin A, which is the EMC virus receptor molecule on virus-nonpermissive human erythrocytes (HeLa cells do not express glycophorin A). These results indicate that EMC virus attachment to permissive human cells is mediated by a cell surface sialoglycoprotein(s) with a molecular mass of 70 kDa.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlish J. D., Lahijani R. S., St Jeor S. C. Identification of a putative cell receptor for human cytomegalovirus. Virology. 1990 Jun;176(2):337–345. doi: 10.1016/0042-6822(90)90003-a. [DOI] [PubMed] [Google Scholar]
- Allaway G. P., Burness A. T. Analysis of the bond between encephalomyocarditis virus and its human erythrocyte receptor by affinity chromatography on virus-sepharose columns. J Gen Virol. 1987 Jul;68(Pt 7):1849–1856. doi: 10.1099/0022-1317-68-7-1849. [DOI] [PubMed] [Google Scholar]
- Allaway G. P., Burness A. T. Site of attachment of encephalomyocarditis virus on human erythrocytes. J Virol. 1986 Sep;59(3):768–770. doi: 10.1128/jvi.59.3.768-770.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel M. A., Burness A. T. The attachment of encephalomyocarditis virus to erythrocytes from several animal species. Virology. 1977 Dec;83(2):428–432. doi: 10.1016/0042-6822(77)90189-1. [DOI] [PubMed] [Google Scholar]
- Anstee D. J., Edwards P. A. Monoclonal antibodies to human erythrocytes. Eur J Immunol. 1982 Mar;12(3):228–232. doi: 10.1002/eji.1830120311. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H., Summers D. F. Purification and properties of HeLa cell plasma membranes. J Biol Chem. 1971 Aug 25;246(16):5162–5175. [PubMed] [Google Scholar]
- Birkenbach M., Tong X., Bradbury L. E., Tedder T. F., Kieff E. Characterization of an Epstein-Barr virus receptor on human epithelial cells. J Exp Med. 1992 Nov 1;176(5):1405–1414. doi: 10.1084/jem.176.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P., Oldstone M. B. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J Virol. 1992 Dec;66(12):7270–7281. doi: 10.1128/jvi.66.12.7270-7281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau W. C., Atwood W. J., Norkin L. C. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J Virol. 1992 Apr;66(4):2037–2045. doi: 10.1128/jvi.66.4.2037-2045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burness A. T., Clothier F. W. Particle weight and other biophysical properties of encephalomyocarditis virus. J Gen Virol. 1970 Mar;6(3):381–393. doi: 10.1099/0022-1317-6-3-381. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Fox S. M., Pardoe I. U. The polypeptide composition of the encephalomyocarditis virus particle. J Gen Virol. 1974 Jun;23(3):225–236. doi: 10.1099/0022-1317-23-3-225. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Pardoe I. U. Chromatofocusing of sialoglycoproteins. J Chromatogr. 1983 Apr 15;259(3):423–432. doi: 10.1016/s0021-9673(01)88030-4. [DOI] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Fields B. N., Greene M. I. Isolation and biochemical characterization of the mammalian reovirus type 3 cell-surface receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1494–1498. doi: 10.1073/pnas.82.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J. Cell surface receptors for picornaviruses. Bioessays. 1986 Dec;5(6):270–274. doi: 10.1002/bies.950050609. [DOI] [PubMed] [Google Scholar]
- Crane S. E., Buzy J., Clements J. E. Identification of cell membrane proteins that bind visna virus. J Virol. 1991 Nov;65(11):6137–6143. doi: 10.1128/jvi.65.11.6137-6143.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea S. A., Bilodeau R., Martineau G. P. Isolation of encephalomyocarditis virus among stillborn and post-weaning pigs in Quebec. Arch Virol. 1991;117(1-2):121–128. doi: 10.1007/BF01310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R., Liu P., Wee L., Butany J., Sole M. J. Verapamil ameliorates the clinical and pathological course of murine myocarditis. J Clin Invest. 1992 Nov;90(5):2022–2030. doi: 10.1172/JCI116082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fotiadis C., Kilpatrick D. R., Lipton H. L. Comparison of the binding characteristics to BHK-21 cells of viruses representing the two Theiler's virus neurovirulence groups. Virology. 1991 May;182(1):365–370. doi: 10.1016/0042-6822(91)90683-3. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Lapidot M., Zakai N., Loyter A. Protein blot analysis of virus receptors: identification and characterization of the Sendai virus receptor. Biochim Biophys Acta. 1986 Mar 27;856(1):19–26. doi: 10.1016/0005-2736(86)90004-0. [DOI] [PubMed] [Google Scholar]
- Hanham C. A., Zhao F., Tignor G. H. Evidence from the anti-idiotypic network that the acetylcholine receptor is a rabies virus receptor. J Virol. 1993 Jan;67(1):530–542. doi: 10.1128/jvi.67.1.530-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A. VCAM-1 is a receptor for encephalomyocarditis virus on murine vascular endothelial cells. J Virol. 1994 Jun;68(6):3453–3458. doi: 10.1128/jvi.68.6.3453-3458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNGEBLUT C. W., KODZA H. Studies on propagation of the Col SK group of viruses in various tissue culture media. Proc Soc Exp Biol Med. 1957 Oct;96(1):133–139. doi: 10.3181/00379727-96-23413. [DOI] [PubMed] [Google Scholar]
- Johns L. D., Flanders K. C., Ranges G. E., Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-beta 1. J Immunol. 1991 Sep 15;147(6):1792–1796. [PubMed] [Google Scholar]
- Jokinen M., Andersson L. C., Gahmberg C. G. Biosynthesis of the major human red cell sialoglycoprotein, glycophorin A. O-Glycosylation. J Biol Chem. 1985 Sep 15;260(20):11314–11321. [PubMed] [Google Scholar]
- Jones C. L., Ehrenfeld E. The effect of poliovirus infection on the translation in vitro of VSV messenger ribonucleoprotein particles. Virology. 1983 Sep;129(2):415–430. doi: 10.1016/0042-6822(83)90180-0. [DOI] [PubMed] [Google Scholar]
- Jones H. W., Jr, McKusick V. A., Harper P. S., Wuu K. D. George Otto Gey. (1899-1970). The HeLa cell and a reappraisal of its origin. Obstet Gynecol. 1971 Dec;38(6):945–949. [PubMed] [Google Scholar]
- Kang Y., Yoon J. W. A genetically determined host factor controlling susceptibility to encephalomyocarditis virus-induced diabetes in mice. J Gen Virol. 1993 Jun;74(Pt 6):1207–1213. doi: 10.1099/0022-1317-74-6-1207. [DOI] [PubMed] [Google Scholar]
- Kassner P. D., Hemler M. E. Interchangeable alpha chain cytoplasmic domains play a positive role in control of cell adhesion mediated by VLA-4, a beta 1 integrin. J Exp Med. 1993 Aug 1;178(2):649–660. doi: 10.1084/jem.178.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. R., Lipton H. L. Predominant binding of Theiler's viruses to a 34-kilodalton receptor protein on susceptible cell lines. J Virol. 1991 Oct;65(10):5244–5249. doi: 10.1128/jvi.65.10.5244-5249.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krah D. L., Crowell R. L. Properties of the deoxycholate-solubilized HeLa cell plasma membrane receptor for binding group B coxsackieviruses. J Virol. 1985 Mar;53(3):867–870. doi: 10.1128/jvi.53.3.867-870.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lentz T. L. The recognition event between virus and host cell receptor: a target for antiviral agents. J Gen Virol. 1990 Apr;71(Pt 4):751–766. doi: 10.1099/0022-1317-71-4-751. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Whiteley N. M. Physical and metabolic requirements for early interaction of poliovirus and human rhinovirus with HeLa cells. J Virol. 1976 Sep;19(3):857–870. doi: 10.1128/jvi.19.3.857-870.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- McClintock P. R., Billups L. C., Notkins A. L. Receptors for encephalomyocarditis virus on murine and human cells. Virology. 1980 Oct 30;106(2):261–272. doi: 10.1016/0042-6822(80)90249-4. [DOI] [PubMed] [Google Scholar]
- Medrano L., Green H. Picornavirus receptors and picornavirus multiplication in human-mouse hybrid cell lines. Virology. 1973 Aug;54(2):515–524. doi: 10.1016/0042-6822(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Miller F. W., Love L. A., Biswas T., McClintock P. R., Notkins A. L., Plotz P. H. Viral and host genetic factors influence encephalomyocarditis virus-induced polymyositis in adult mice. Arthritis Rheum. 1987 May;30(5):549–556. doi: 10.1002/art.1780300509. [DOI] [PubMed] [Google Scholar]
- Pardoe I. U., Grewal K. K., Baldeh M. P., Hamid J., Burness A. T. Persistent infection of K562 cells by encephalomyocarditis virus. J Virol. 1990 Dec;64(12):6040–6044. doi: 10.1128/jvi.64.12.6040-6044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier R. P., Ohye R. G., Vanbuskirk A., Sedmak D. D., Kincade P., Ferguson R. M., Orosz C. G. Importance of endothelial VCAM-1 for inflammatory leukocytic infiltration in vivo. J Immunol. 1992 Oct 1;149(7):2473–2481. [PubMed] [Google Scholar]
- Racaniello V. R. Cell receptors for picornaviruses. Curr Top Microbiol Immunol. 1990;161:1–22. doi: 10.1007/978-3-642-75602-3_1. [DOI] [PubMed] [Google Scholar]
- Shu H. B., Agranoff A. B., Nabel E. G., Leung K., Duckett C. S., Neish A. S., Collins T., Nabel G. J. Differential regulation of vascular cell adhesion molecule 1 gene expression by specific NF-kappa B subunits in endothelial and epithelial cells. Mol Cell Biol. 1993 Oct;13(10):6283–6289. doi: 10.1128/mcb.13.10.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. E., Adamany A. M., Blumenfeld O. O. Glycophorins of human erythroleukemic K562 cells. Arch Biochem Biophys. 1987 Jul;256(1):285–294. doi: 10.1016/0003-9861(87)90448-6. [DOI] [PubMed] [Google Scholar]
- Tavakkol A., Burness A. T. Evidence for a direct role for sialic acid in the attachment of encephalomyocarditis virus to human erythrocytes. Biochemistry. 1990 Nov 27;29(47):10684–10690. doi: 10.1021/bi00499a016. [DOI] [PubMed] [Google Scholar]
- Taylor H. P., Cooper N. R. The human cytomegalovirus receptor on fibroblasts is a 30-kilodalton membrane protein. J Virol. 1990 Jun;64(6):2484–2490. doi: 10.1128/jvi.64.6.2484-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R. B. The prevalence of encephalomyocarditis virus neutralizing antibodies among various human populations. Am J Trop Med Hyg. 1978 Jan;27(1 Pt 1):144–149. doi: 10.4269/ajtmh.1978.27.144. [DOI] [PubMed] [Google Scholar]
- Tomassini J. E., Graham D., DeWitt C. M., Lineberger D. W., Rodkey J. A., Colonno R. J. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4907–4911. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]