Abstract
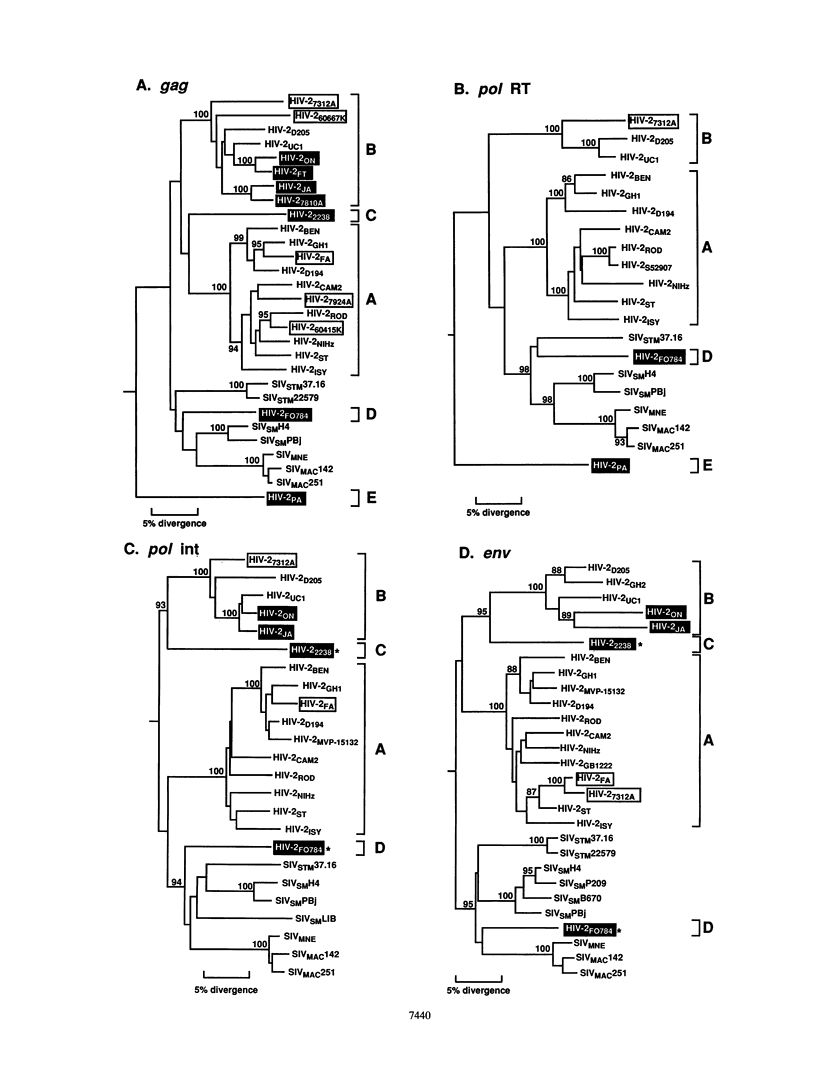
The virulence properties of human immunodeficiency virus type 2 (HIV-2) are known to vary significantly and to range from relative attenuation in certain individuals to high-level pathogenicity in others. These differences in clinical manifestations may, at least in part, be determined by genetic differences among infecting virus strains. Evaluation of the full spectrum of HIV-2 genetic diversity is thus a necessary first step towards understanding its molecular epidemiology, natural history of infection, and biological diversity. In this study, we have used nested PCR techniques to amplify viral sequences from the DNA of uncultured peripheral blood mononuclear cells from 12 patients with HIV-2 seroreactivity. Sequence analysis of four nonoverlapping genomic regions allowed a comprehensive analysis of HIV-2 phylogeny. The results revealed (i) the existence of five distinct and roughly equidistant evolutionary lineages of HIV-2 which, by analogy with HIV-1, have been termed sequence subtypes A to E; (ii) evidence for a mosaic HIV-2 genome, indicating that coinfection with genetically divergent strains and recombination can occur in HIV-2-infected individuals; and (iii) evidence supporting the conclusion that some of the HIV-2 subtypes may have arisen from independent introductions of genetically diverse sooty mangabey viruses into the human population. Importantly, only a subset of HIV-2 strains replicated in culture: all subtype A viruses grew to high titers, but attempts to isolate representatives of subtypes C, D, and E, as well as the majority of subtype B viruses, remained unsuccessful. Infection with all five viral subtypes was detectable by commercially available serological (Western immunoblot) assays, despite intersubtype sequence differences of up to 25% in the gag, pol, and env regions. These results indicate that the genetic and biological diversity of HIV-2 is far greater than previously appreciated and suggest that there may be subtype-specific differences in virus biology. Systematic natural history studies are needed to determine whether this heterogeneity has clinical relevance and whether the various HIV-2 subtypes differ in their in vivo pathogenicity.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Nauclér A., Böttiger B., Broliden P. A., Albino P., Ouattara S. A., Björkegren C., Valentin A., Biberfeld G., Fenyö E. M. Replicative capacity of HIV-2, like HIV-1, correlates with severity of immunodeficiency. AIDS. 1990 Apr;4(4):291–295. doi: 10.1097/00002030-199004000-00002. [DOI] [PubMed] [Google Scholar]
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Allan J. S., Short M., Taylor M. E., Su S., Hirsch V. M., Johnson P. R., Shaw G. M., Hahn B. H. Species-specific diversity among simian immunodeficiency viruses from African green monkeys. J Virol. 1991 Jun;65(6):2816–2828. doi: 10.1128/jvi.65.6.2816-2828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelle R., Bletry O., Baglin A. C., Brun-Vezinet F., Rey M. A., Godeau P. Long incubation period for HIV-2 infection. Lancet. 1987 Mar 21;1(8534):688–689. doi: 10.1016/s0140-6736(87)90454-5. [DOI] [PubMed] [Google Scholar]
- Andreasson P. A., Dias F., Nauclér A., Andersson S., Biberfeld G. A prospective study of vertical transmission of HIV-2 in Bissau, Guinea-Bissau. AIDS. 1993 Jul;7(7):989–993. [PubMed] [Google Scholar]
- Barin F., M'Boup S., Denis F., Kanki P., Allan J. S., Lee T. H., Essex M. Serological evidence for virus related to simian T-lymphotropic retrovirus III in residents of west Africa. Lancet. 1985 Dec 21;2(8469-70):1387–1389. doi: 10.1016/s0140-6736(85)92556-5. [DOI] [PubMed] [Google Scholar]
- Barnett S. W., Quiroga M., Werner A., Dina D., Levy J. A. Distinguishing features of an infectious molecular clone of the highly divergent and noncytopathic human immunodeficiency virus type 2 UC1 strain. J Virol. 1993 Feb;67(2):1006–1014. doi: 10.1128/jvi.67.2.1006-1014.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeri E., Giri A., Lillo F., Ferrari G., Varnier O. E., Ferro A., Sabbatani S., Saxinger W. C., Franchini G. In vivo genetic variability of the human immunodeficiency virus type 2 V3 region. J Virol. 1992 Jul;66(7):4546–4550. doi: 10.1128/jvi.66.7.4546-4550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman F. D., Engelman A., Palmer I., Wingfield P., Craigie R. Domains of the integrase protein of human immunodeficiency virus type 1 responsible for polynucleotidyl transfer and zinc binding. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3428–3432. doi: 10.1073/pnas.90.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro B. A., Barnett S. W., Evans L. A., Moreau J., Odehouri K., Levy J. A. Biologic heterogeneity of human immunodeficiency virus type 2 (HIV-2) strains. Virology. 1990 Oct;178(2):527–534. doi: 10.1016/0042-6822(90)90350-z. [DOI] [PubMed] [Google Scholar]
- Clark S. J., Saag M. S., Decker W. D., Campbell-Hill S., Roberson J. L., Veldkamp P. J., Kappes J. C., Hahn B. H., Shaw G. M. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991 Apr 4;324(14):954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Clavel F., Mansinho K., Chamaret S., Guetard D., Favier V., Nina J., Santos-Ferreira M. O., Champalimaud J. L., Montagnier L. Human immunodeficiency virus type 2 infection associated with AIDS in West Africa. N Engl J Med. 1987 May 7;316(19):1180–1185. doi: 10.1056/NEJM198705073161903. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Davies J. F., 2nd, Hostomska Z., Hostomsky Z., Jordan S. R., Matthews D. A. Crystal structure of the ribonuclease H domain of HIV-1 reverse transcriptase. Science. 1991 Apr 5;252(5002):88–95. doi: 10.1126/science.1707186. [DOI] [PubMed] [Google Scholar]
- De Cock K. M., Adjorlolo G., Ekpini E., Sibailly T., Kouadio J., Maran M., Brattegaard K., Vetter K. M., Doorly R., Gayle H. D. Epidemiology and transmission of HIV-2. Why there is no HIV-2 pandemic. JAMA. 1993 Nov 3;270(17):2083–2086. doi: 10.1001/jama.270.17.2083. [DOI] [PubMed] [Google Scholar]
- De Cock K. M., Barrere B., Diaby L., Lafontaine M. F., Gnaore E., Porter A., Pantobe D., Lafontant G. C., Dago-Akribi A., Ette M. AIDS--the leading cause of adult death in the West African City of Abidjan, Ivory Coast. Science. 1990 Aug 17;249(4970):793–796. doi: 10.1126/science.2167515. [DOI] [PubMed] [Google Scholar]
- De Cock K. M., Odehouri K., Colebunders R. L., Adjorlolo G., Lafontaine M. F., Porter A., Gnaore E., Diaby L., Moreau J., Heyward W. L. A comparison of HIV-1 and HIV-2 infections in hospitalized patients in Abidjan, Côte d'Ivoire. AIDS. 1990 May;4(5):443–448. doi: 10.1097/00002030-199005000-00010. [DOI] [PubMed] [Google Scholar]
- Delwart E. L., Shpaer E. G., Louwagie J., McCutchan F. E., Grez M., Rübsamen-Waigmann H., Mullins J. I. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993 Nov 19;262(5137):1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- Dietrich U., Adamski M., Kreutz R., Seipp A., Kühnel H., Rübsamen-Waigmann H. A highly divergent HIV-2-related isolate. Nature. 1989 Dec 21;342(6252):948–950. doi: 10.1038/342948a0. [DOI] [PubMed] [Google Scholar]
- Donnelly C., Leisenring W., Kanki P., Awerbuch T., Sandberg S. Comparison of transmission rates of HIV-1 and HIV-2 in a cohort of prostitutes in Senegal. Bull Math Biol. 1993;55(4):731–743. doi: 10.1007/BF02460671. [DOI] [PubMed] [Google Scholar]
- Dufoort G., Couroucé A. M., Ancelle-Park R., Bletry O. No clinical signs 14 years after HIV-2 transmission via blood transfusion. Lancet. 1988 Aug 27;2(8609):510–510. doi: 10.1016/s0140-6736(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Evans L. A., Moreau J., Odehouri K., Legg H., Barboza A., Cheng-Mayer C., Levy J. A. Characterization of a noncytopathic HIV-2 strain with unusual effects on CD4 expression. Science. 1988 Jun 10;240(4858):1522–1525. doi: 10.1126/science.2836951. [DOI] [PubMed] [Google Scholar]
- Faulkner D. V., Jurka J. Multiple aligned sequence editor (MASE). Trends Biochem Sci. 1988 Aug;13(8):321–322. doi: 10.1016/0968-0004(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Franchini G., Fargnoli K. A., Giombini F., Jagodzinski L., De Rossi A., Bosch M., Biberfeld G., Fenyo E. M., Albert J., Gallo R. C. Molecular and biological characterization of a replication competent human immunodeficiency type 2 (HIV-2) proviral clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2433–2437. doi: 10.1073/pnas.86.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R., Ball J. M., Garry R. F., Griffin M. C., Montelaro R. C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989 Aug;5(4):431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- Gao F., Yue L., White A. T., Pappas P. G., Barchue J., Hanson A. P., Greene B. M., Sharp P. M., Shaw G. M., Hahn B. H. Human infection by genetically diverse SIVSM-related HIV-2 in west Africa. Nature. 1992 Aug 6;358(6386):495–499. doi: 10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- Grez M., Dietrich U., Balfe P., von Briesen H., Maniar J. K., Mahambre G., Delwart E. L., Mullins J. I., Rübsamen-Waigmann H. Genetic analysis of human immunodeficiency virus type 1 and 2 (HIV-1 and HIV-2) mixed infections in India reveals a recent spread of HIV-1 and HIV-2 from a single ancestor for each of these viruses. J Virol. 1994 Apr;68(4):2161–2168. doi: 10.1128/jvi.68.4.2161-2168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Gürtler L. G., Hauser P. H., Eberle J., von Brunn A., Knapp S., Zekeng L., Tsague J. M., Kaptue L. A new subtype of human immunodeficiency virus type 1 (MVP-5180) from Cameroon. J Virol. 1994 Mar;68(3):1581–1585. doi: 10.1128/jvi.68.3.1581-1585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A., Tsujimoto H., Maki N., Ishikawa K., Miura T., Fukasawa M., Miki K., Hayami M. Genomic divergence of HIV-2 from Ghana. AIDS Res Hum Retroviruses. 1989 Dec;5(6):593–604. doi: 10.1089/aid.1989.5.593. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hirsch V. M., Olmsted R. A., Murphey-Corb M., Purcell R. H., Johnson P. R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989 Jun 1;339(6223):389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Lee S., Moudgil T., Kerndt P. R. HIV-2 in Los Angeles. AIDS. 1990 Dec;4(12):1301–1302. doi: 10.1097/00002030-199012000-00028. [DOI] [PubMed] [Google Scholar]
- Huet T., Cheynier R., Meyerhans A., Roelants G., Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990 May 24;345(6273):356–359. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., Travers K. U., MBoup S., Hsieh C. C., Marlink R. G., Gueye-NDiaye A., Siby T., Thior I., Hernandez-Avila M., Sankalé J. L. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994 Apr 16;343(8903):943–946. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Katahira J., Fukasawa M., Sakuragi J., Ishikawa K., Nakai M., Mingle J. A., Osei-Kwasi M., Netty V. B., Akari H. Isolation and characterization of a highly divergent HIV-2[GH-2]: generation of an infectious molecular clone and functional analysis of its rev-responsive element in response to primate retrovirus transactivators (Rev and Rex). Virology. 1992 Jun;188(2):850–853. doi: 10.1016/0042-6822(92)90540-6. [DOI] [PubMed] [Google Scholar]
- Khabbaz R. F., Heneine W., George J. R., Parekh B., Rowe T., Woods T., Switzer W. M., McClure H. M., Murphey-Corb M., Folks T. M. Brief report: infection of a laboratory worker with simian immunodeficiency virus. N Engl J Med. 1994 Jan 20;330(3):172–177. doi: 10.1056/NEJM199401203300304. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Galvin T. A., Lowenstine L. J., Jennings M. B., Gardner M. B., Buckler C. E. A highly divergent simian immunodeficiency virus (SIVstm) recovered from stored stump-tailed macaque tissues. J Virol. 1991 Dec;65(12):7061–7065. doi: 10.1128/jvi.65.12.7061-7065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F., Jentsch K. D., Stuke A., Mous J., Hunsmann G. Genomic divergence of an HIV-2 from a German AIDS patient probably infected in Mali. AIDS. 1990 Sep;4(9):847–857. doi: 10.1097/00002030-199009000-00003. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt L. A., Wang J., Friedman J. M., Rice P. A., Steitz T. A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- Kong L. I., Lee S. W., Kappes J. C., Parkin J. S., Decker D., Hoxie J. A., Hahn B. H., Shaw G. M. West African HIV-2-related human retrovirus with attenuated cytopathicity. Science. 1988 Jun 10;240(4858):1525–1529. doi: 10.1126/science.3375832. [DOI] [PubMed] [Google Scholar]
- Kumar P., Hui H. X., Kappes J. C., Haggarty B. S., Hoxie J. A., Arya S. K., Shaw G. M., Hahn B. H. Molecular characterization of an attenuated human immunodeficiency virus type 2 isolate. J Virol. 1990 Feb;64(2):890–901. doi: 10.1128/jvi.64.2.890-901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi K., Conway B., Cunningham S., Berson A., Evans C., Iversen A. K., Colvin D., Gallo M. V., Coutre S., Shpaer E. G. Human immunodeficiency virus type 1 envelope gene structure and diversity in vivo and after cocultivation in vitro. J Virol. 1992 Feb;66(2):875–885. doi: 10.1128/jvi.66.2.875-885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. H., Tanimura M., Sharp P. M. Rates and dates of divergence between AIDS virus nucleotide sequences. Mol Biol Evol. 1988 Jul;5(4):313–330. doi: 10.1093/oxfordjournals.molbev.a040503. [DOI] [PubMed] [Google Scholar]
- Li Y., Hui H., Burgess C. J., Price R. W., Sharp P. M., Hahn B. H., Shaw G. M. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992 Nov;66(11):6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson A. R., Buchbinder S. P., Sheppard H. W., Mawle A. C., Wilber J. C., Stanley M., Hart C. E., Hessol N. A., Holmberg S. D. Long-term human immunodeficiency virus infection in asymptomatic homosexual and bisexual men with normal CD4+ lymphocyte counts: immunologic and virologic characteristics. J Infect Dis. 1991 May;163(5):959–965. doi: 10.1093/infdis/163.5.959. [DOI] [PubMed] [Google Scholar]
- Loussert-Ajaka I., Ly T. D., Chaix M. L., Ingrand D., Saragosti S., Couroucé A. M., Brun-Vézinet F., Simon F. HIV-1/HIV-2 seronegativity in HIV-1 subtype O infected patients. Lancet. 1994 Jun 4;343(8910):1393–1394. doi: 10.1016/s0140-6736(94)92524-0. [DOI] [PubMed] [Google Scholar]
- Louwagie J., McCutchan F. E., Peeters M., Brennan T. P., Sanders-Buell E., Eddy G. A., van der Groen G., Fransen K., Gershy-Damet G. M., Deleys R. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993 Jun;7(6):769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- Markovitz D. M. Infection with the human immunodeficiency virus type 2. Ann Intern Med. 1993 Feb 1;118(3):211–218. doi: 10.7326/0003-4819-118-3-199302010-00010. [DOI] [PubMed] [Google Scholar]
- Marlink R. G., Ricard D., M'Boup S., Kanki P. J., Romet-Lemonne J. L., N'Doye I., Diop K., Simpson M. A., Greco F., Chou M. J. Clinical, hematologic, and immunologic cross-sectional evaluation of individuals exposed to human immunodeficiency virus type-2 (HIV-2). AIDS Res Hum Retroviruses. 1988 Apr;4(2):137–148. doi: 10.1089/aid.1988.4.137. [DOI] [PubMed] [Google Scholar]
- Marx P. A., Li Y., Lerche N. W., Sutjipto S., Gettie A., Yee J. A., Brotman B. H., Prince A. M., Hanson A., Webster R. G. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a west African pet sooty mangabey. J Virol. 1991 Aug;65(8):4480–4485. doi: 10.1128/jvi.65.8.4480-4485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers G., MacInnes K., Korber B. The emergence of simian/human immunodeficiency viruses. AIDS Res Hum Retroviruses. 1992 Mar;8(3):373–386. doi: 10.1089/aid.1992.8.373. [DOI] [PubMed] [Google Scholar]
- Nauclér A., Albino P., Andersson S., Paolo da Silva A., Linder H., Andreasson P. A., Biberfeld G. Clinical and immunological follow-up of previously hospitalized HIV-2 seropositive patients in Bissau, Guinea-Bissau. Scand J Infect Dis. 1992;24(6):725–731. doi: 10.3109/00365549209062457. [DOI] [PubMed] [Google Scholar]
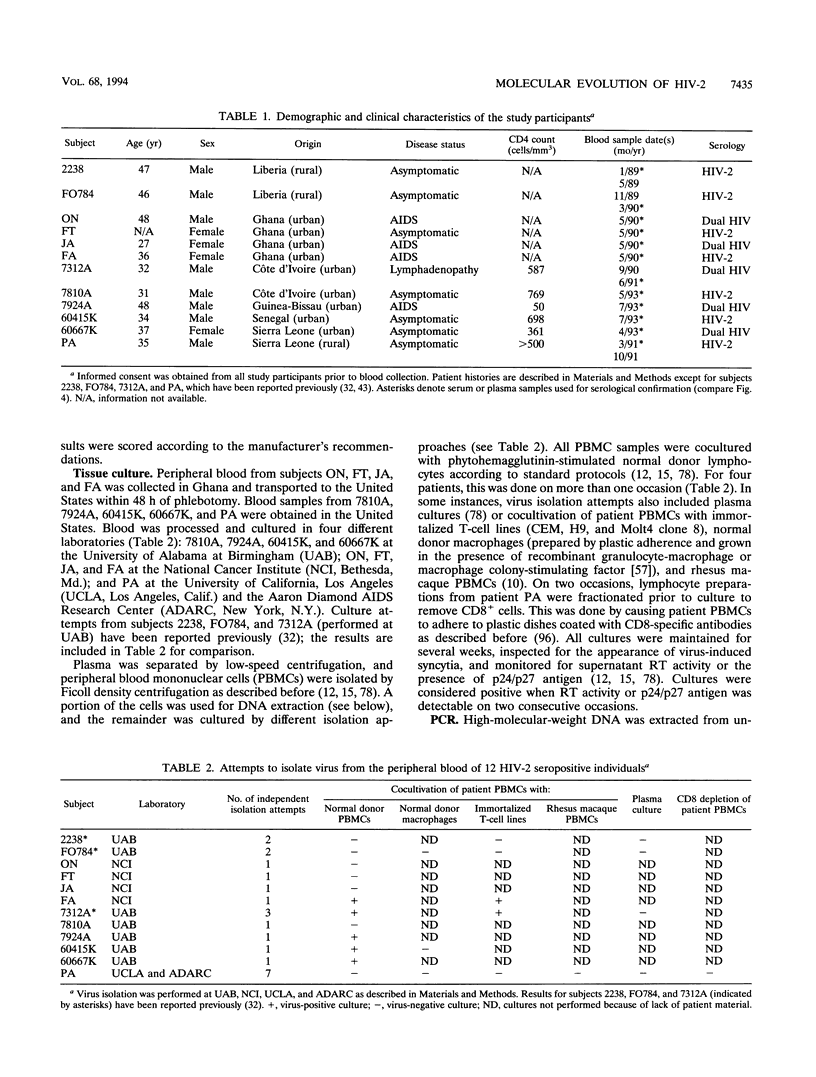
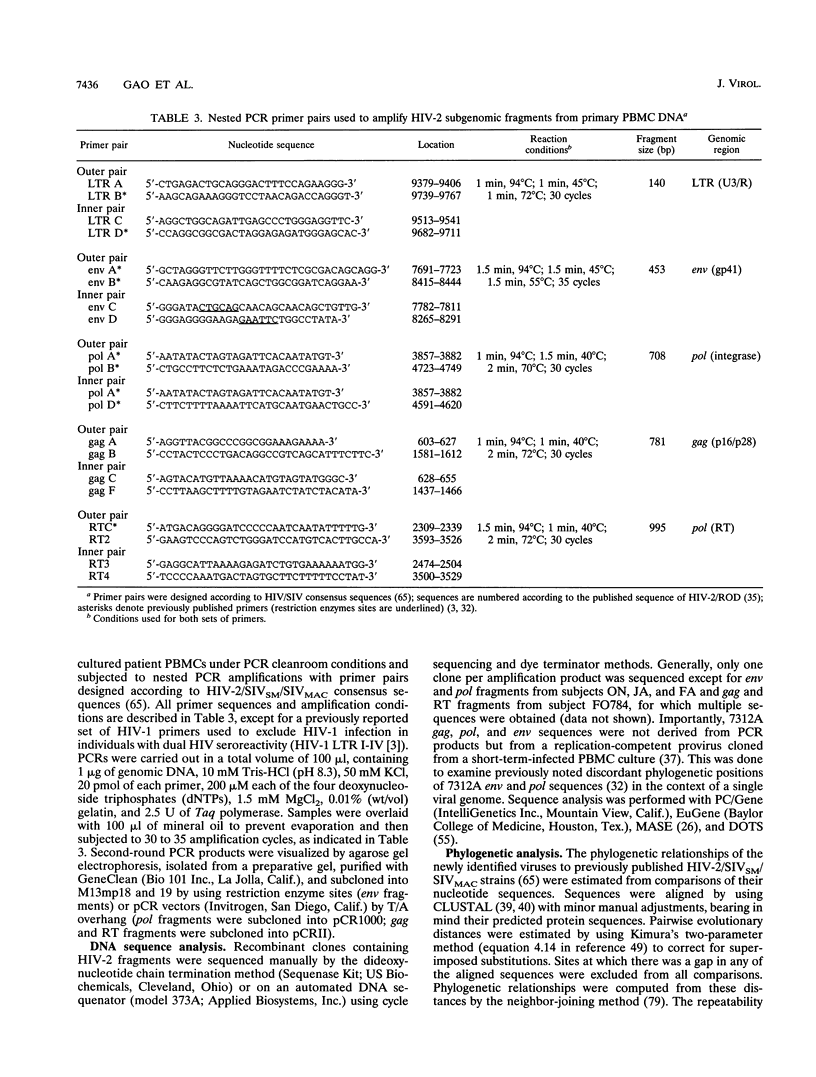
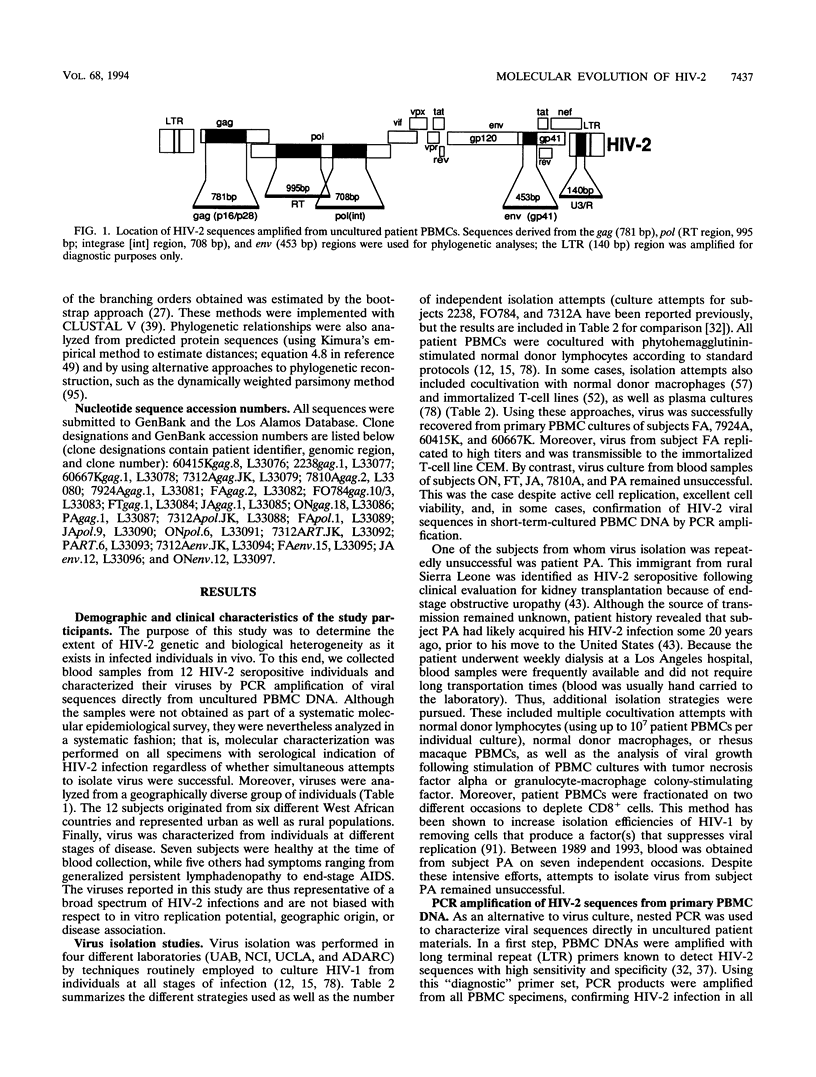
- Nauclér A., Albino P., Da Silva A. P., Andreasson P. A., Andersson S., Biberfeld G. HIV-2 infection in hospitalized patients in Bissau, Guinea-Bissau. AIDS. 1991 Mar;5(3):301–304. doi: 10.1097/00002030-199103000-00009. [DOI] [PubMed] [Google Scholar]
- Novembre F. J., Hirsch V. M., McClure H. M., Fultz P. N., Johnson P. R. SIV from stump-tailed macaques: molecular characterization of a highly transmissible primate lentivirus. Virology. 1992 Feb;186(2):783–787. doi: 10.1016/0042-6822(92)90047-s. [DOI] [PubMed] [Google Scholar]
- Peeters M., Fransen K., Delaporte E., Van den Haesevelde M., Gershy-Damet G. M., Kestens L., van der Groen G., Piot P. Isolation and characterization of a new chimpanzee lentivirus (simian immunodeficiency virus isolate cpz-ant) from a wild-captured chimpanzee. AIDS. 1992 May;6(5):447–451. doi: 10.1097/00002030-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Pepin J., Morgan G., Dunn D., Gevao S., Mendy M., Gaye I., Scollen N., Tedder R., Whittle H. HIV-2-induced immunosuppression among asymptomatic West African prostitutes: evidence that HIV-2 is pathogenic, but less so than HIV-1. AIDS. 1991 Oct;5(10):1165–1172. [PubMed] [Google Scholar]
- Pfützner A., Dietrich U., von Eichel U., von Briesen H., Brede H. D., Maniar J. K., Rübsamen-Waigmann H. HIV-1 and HIV-2 infections in a high-risk population in Bombay, India: evidence for the spread of HIV-2 and presence of a divergent HIV-1 subtype. J Acquir Immune Defic Syndr. 1992 Oct;5(10):972–977. [PubMed] [Google Scholar]
- Poulsen A. G., Aaby P., Gottschau A., Kvinesdal B. B., Dias F., Mølbak K., Lauritzen E. HIV-2 infection in Bissau, West Africa, 1987-1989: incidence, prevalences, and routes of transmission. J Acquir Immune Defic Syndr. 1993 Aug;6(8):941–948. [PubMed] [Google Scholar]
- Quinn T. C. Population migration and the spread of types 1 and 2 human immunodeficiency viruses. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2407–2414. doi: 10.1073/pnas.91.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I., Marlink R., Kanki P., M'Boup S., Essex M. HIV-2 link to AIDS in West Africa. J Acquir Immune Defic Syndr. 1990;3(3):220–230. [PubMed] [Google Scholar]
- Rübsamen-Waigmann H., Briesen H. V., Maniar J. K., Rao P. K., Scholz C., Pfützner A. Spread of HIV-2 in India. Lancet. 1991 Mar 2;337(8740):550–551. doi: 10.1016/0140-6736(91)91333-p. [DOI] [PubMed] [Google Scholar]
- Saag M. S., Crain M. J., Decker W. D., Campbell-Hill S., Robinson S., Brown W. E., Leuther M., Whitley R. J., Hahn B. H., Shaw G. M. High-level viremia in adults and children infected with human immunodeficiency virus: relation to disease stage and CD4+ lymphocyte levels. J Infect Dis. 1991 Jul;164(1):72–80. doi: 10.1093/infdis/164.1.72. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira M. O., Cohen T., Lourenço M. H., Almeida M. J., Chamaret S., Montagnier L. A study of seroprevalence of HIV-1 and HIV-2 in six provinces of People's Republic of Angola: clues to the spread of HIV infection. J Acquir Immune Defic Syndr. 1990;3(8):780–786. [PubMed] [Google Scholar]
- Schulz T. F., Whitby D., Hoad J. G., Corrah T., Whittle H., Weiss R. A. Biological and molecular variability of human immunodeficiency virus type 2 isolates from The Gambia. J Virol. 1990 Oct;64(10):5177–5182. doi: 10.1128/jvi.64.10.5177-5182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F., Matheron S., Tamalet C., Loussert-Ajaka I., Bartczak S., Pépin J. M., Dhiver C., Gamba E., Elbim C., Gastaut J. A. Cellular and plasma viral load in patients infected with HIV-2. AIDS. 1993 Nov;7(11):1411–1417. doi: 10.1097/00002030-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Talbott R., Kraus G., Looney D., Wong-Staal F. Mapping the determinants of human immunodeficiency virus 2 for infectivity, replication efficiency, and cytopathicity. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4226–4230. doi: 10.1073/pnas.90.9.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristem M., Hill F., Karpas A. Nucleotide sequence of a Guinea-Bissau-derived human immunodeficiency virus type 2 proviral clone (HIV-2CAM2). J Gen Virol. 1991 Mar;72(Pt 3):721–724. doi: 10.1099/0022-1317-72-3-721. [DOI] [PubMed] [Google Scholar]
- Tözsér J., Bláha I., Copeland T. D., Wondrak E. M., Oroszlan S. Comparison of the HIV-1 and HIV-2 proteinases using oligopeptide substrates representing cleavage sites in Gag and Gag-Pol polyproteins. FEBS Lett. 1991 Apr 9;281(1-2):77–80. doi: 10.1016/0014-5793(91)80362-7. [DOI] [PubMed] [Google Scholar]
- Vanden Haesevelde M., Decourt J. L., De Leys R. J., Vanderborght B., van der Groen G., van Heuverswijn H., Saman E. Genomic cloning and complete sequence analysis of a highly divergent African human immunodeficiency virus isolate. J Virol. 1994 Mar;68(3):1586–1596. doi: 10.1128/jvi.68.3.1586-1596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield J. K., Jablonski S. A., Morrow C. D. In vitro enzymatic activity of human immunodeficiency virus type 1 reverse transcriptase mutants in the highly conserved YMDD amino acid motif correlates with the infectious potential of the proviral genome. J Virol. 1992 Nov;66(11):6806–6812. doi: 10.1128/jvi.66.11.6806-6812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Whittle H., Egboga A., Todd J., Corrah T., Wilkins A., Demba E., Morgan G., Rolfe M., Berry N., Tedder R. Clinical and laboratory predictors of survival in Gambian patients with symptomatic HIV-1 or HIV-2 infection. AIDS. 1992 Jul;6(7):685–689. doi: 10.1097/00002030-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Whittle H., Egboga A., Todd J., Morgan G., Rolfe M., Sabally S., Wilkins A., Corrah T. Immunological responses of Gambians in relation to clinical stage of HIV-2 disease. Clin Exp Immunol. 1993 Jul;93(1):45–50. doi: 10.1111/j.1365-2249.1993.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A., Ricard D., Todd J., Whittle H., Dias F., Paulo Da Silva A. The epidemiology of HIV infection in a rural area of Guinea-Bissau. AIDS. 1993 Aug;7(8):1119–1122. doi: 10.1097/00002030-199308000-00015. [DOI] [PubMed] [Google Scholar]
- Williams P. L., Fitch W. M. Phylogeny determination using dynamically weighted parsimony method. Methods Enzymol. 1990;183:615–626. doi: 10.1016/0076-6879(90)83040-g. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagury J. F., Franchini G., Reitz M., Collalti E., Starcich B., Hall L., Fargnoli K., Jagodzinski L., Guo H. G., Laure F. Genetic variability between isolates of human immunodeficiency virus (HIV) type 2 is comparable to the variability among HIV type 1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5941–5945. doi: 10.1073/pnas.85.16.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]