Abstract
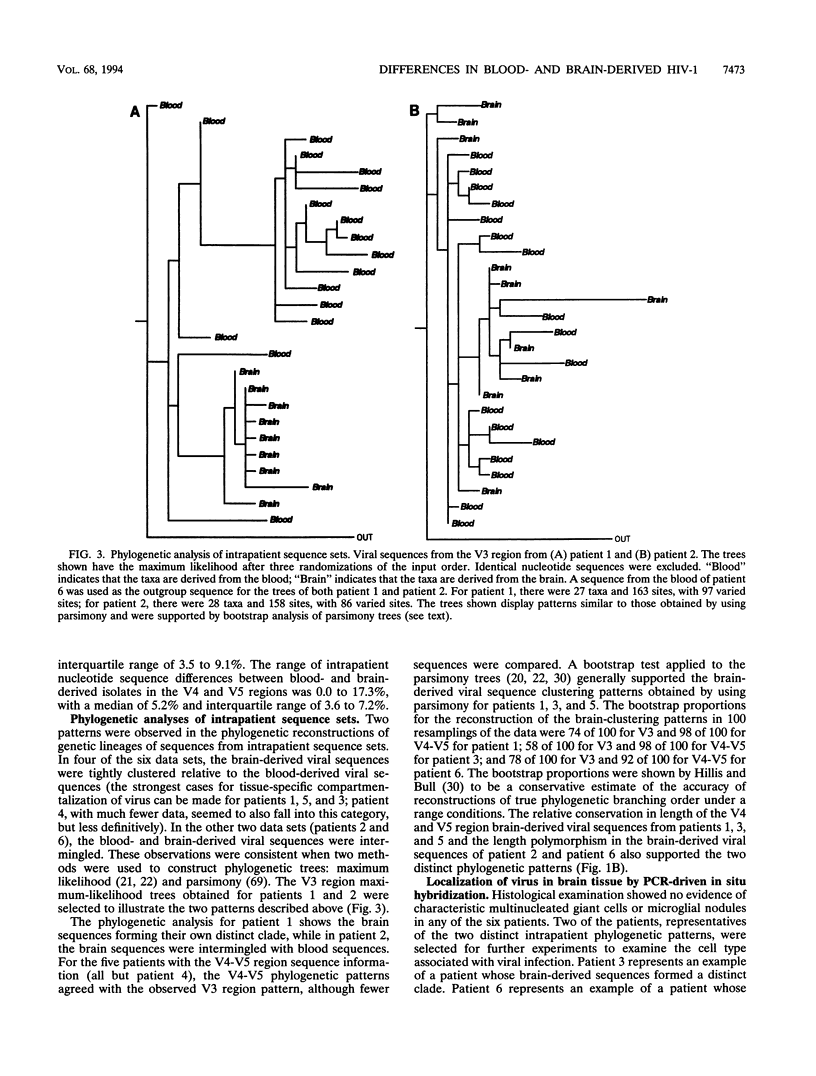
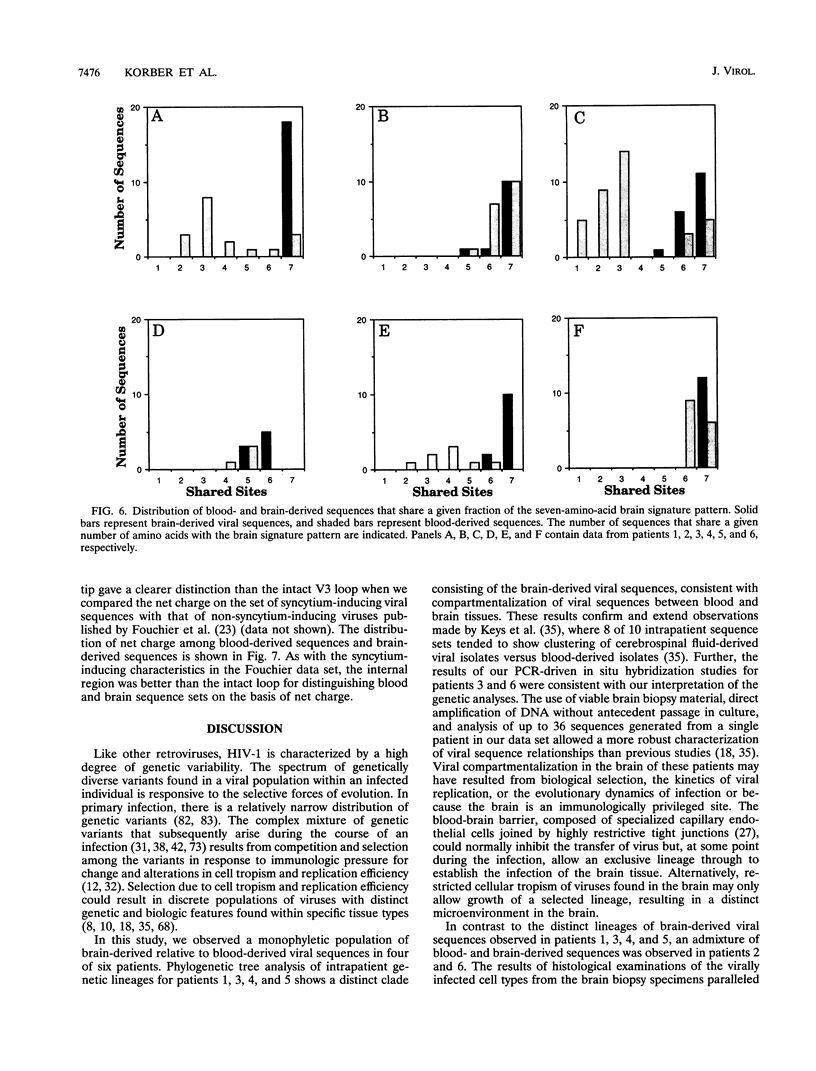
Human immunodeficiency virus type 1 (HIV-1) sequences were generated from blood and from brain tissue obtained by stereotactic biopsy from six patients undergoing a diagnostic neurosurgical procedure. Proviral DNA was directly amplified by nested PCR, and 8 to 36 clones from each sample were sequenced. Phylogenetic analysis of intrapatient envelope V3-V5 region HIV-1 DNA sequence sets revealed that brain viral sequences were clustered relative to the blood viral sequences, suggestive of tissue-specific compartmentalization of the virus in four of the six cases. In the other two cases, the blood and brain virus sequences were intermingled in the phylogenetic analyses, suggesting trafficking of virus between the two tissues. Slide-based PCR-driven in situ hybridization of two of the patients' brain biopsy samples confirmed our interpretation of the intrapatient phylogenetic analyses. Interpatient V3 region brain-derived sequence distances were significantly less than blood-derived sequence distances. Relative to the tip of the loop, the set of brain-derived viral sequences had a tendency towards negative or neutral charge compared with the set of blood-derived viral sequences. Entropy calculations were used as a measure of the variability at each position in alignments of blood and brain viral sequences. A relatively conserved set of positions were found, with a significantly lower entropy in the brain-than in the blood-derived viral sequences. These sites constitute a brain "signature pattern," or a noncontiguous set of amino acids in the V3 region conserved in viral sequences derived from brain tissue. This brain-derived signature pattern was also well preserved among isolates previously characterized in vitro as macrophage tropic. Macrophage-monocyte tropism may be the biological constraint that results in the conservation of the viral brain signature pattern.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andeweg A. C., Leeflang P., Osterhaus A. D., Bosch M. L. Both the V2 and V3 regions of the human immunodeficiency virus type 1 surface glycoprotein functionally interact with other envelope regions in syncytium formation. J Virol. 1993 Jun;67(6):3232–3239. doi: 10.1128/jvi.67.6.3232-3239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Sisler J. R., Earl P. L. Human immunodeficiency virus type 1 envelope glycoprotein molecules containing membrane fusion-impairing mutations in the V3 region efficiently undergo soluble CD4-stimulated gp120 release. J Virol. 1992 Oct;66(10):6208–6212. doi: 10.1128/jvi.66.10.6208-6212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron L., Sullivan N., Sodroski J. Target cell-specific determinants of membrane fusion within the human immunodeficiency virus type 1 gp120 third variable region and gp41 amino terminus. J Virol. 1992 Apr;66(4):2389–2397. doi: 10.1128/jvi.66.4.2389-2397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M. T., Simpson G. R., Cann A. J., Johnson M. A., Weiss R. A. A single amino acid substitution in the V1 loop of human immunodeficiency virus type 1 gp120 alters cellular tropism. J Virol. 1993 Jun;67(6):3649–3652. doi: 10.1128/jvi.67.6.3649-3652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew B. J. HIV-1-related neurological disease. J Acquir Immune Defic Syndr. 1993;6 (Suppl 1):S10–S15. [PubMed] [Google Scholar]
- Budka H., Wiley C. A., Kleihues P., Artigas J., Asbury A. K., Cho E. S., Cornblath D. R., Dal Canto M. C., DeGirolami U., Dickson D. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991 Apr;1(3):143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Homsy J., Evans L. A., Levy J. A. Identification of human immunodeficiency virus subtypes with distinct patterns of sensitivity to serum neutralization. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2815–2819. doi: 10.1073/pnas.85.8.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J. T., Rosenblum M. L., McHugh T., Stites D. P., Levy J. A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Weiss C., Seto D., Levy J. A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong J. J., De Ronde A., Keulen W., Tersmette M., Goudsmit J. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J Virol. 1992 Nov;66(11):6777–6780. doi: 10.1128/jvi.66.11.6777-6780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delassus S., Cheynier R., Wain-Hobson S. Nonhomogeneous distribution of human immunodeficiency virus type 1 proviruses in the spleen. J Virol. 1992 Sep;66(9):5642–5645. doi: 10.1128/jvi.66.9.5642-5645.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks R. W., Van Gijlswijk R. P., Vooijs M. A., Smit A. B., Bogerd J., van Minnen J., Raap A. K., Van der Ploeg M. 3'-end fluorochromized and haptenized oligonucleotides as in situ hybridization probes for multiple, simultaneous RNA detection. Exp Cell Res. 1991 Jun;194(2):310–315. doi: 10.1016/0014-4827(91)90370-a. [DOI] [PubMed] [Google Scholar]
- Dirks R. W., van Gijlswijk R. P., Tullis R. H., Smit A. B., van Minnen J., van der Ploeg M., Raap A. K. Simultaneous detection of different mRNA sequences coding for neuropeptide hormones by double in situ hybridization using FITC- and biotin-labeled oligonucleotides. J Histochem Cytochem. 1990 Apr;38(4):467–473. doi: 10.1177/38.4.2108203. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Beneke J., Till M., Wolinsky S., Ribas J. L., Burke A., Haase A. T. Analysis of human immunodeficiency virus-infected tissues by amplification and in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. G., Kuiken C., Blumberg B. M., Hartman S., Sharer L. R., Clement M., Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology. 1991 Feb;180(2):583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- Faulkner D. V., Jurka J. Multiple aligned sequence editor (MASE). Trends Biochem Sci. 1988 Aug;13(8):321–322. doi: 10.1016/0968-0004(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Fouchier R. A., Groenink M., Kootstra N. A., Tersmette M., Huisman H. G., Miedema F., Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992 May;66(5):3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Identification of the principal neutralizing determinant of human immunodeficiency virus type 1 as a fusion domain. J Virol. 1991 Jan;65(1):190–194. doi: 10.1128/jvi.65.1.190-194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Risser R. Identification of conserved residues in the human immunodeficiency virus type 1 principal neutralizing determinant that are involved in fusion. AIDS Res Hum Retroviruses. 1991 Oct;7(10):807–811. doi: 10.1089/aid.1991.7.807. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Betz A. L. The blood-brain barrier. Sci Am. 1986 Sep;255(3):74–83. doi: 10.1038/scientificamerican0986-74. [DOI] [PubMed] [Google Scholar]
- Groenink M., Fouchier R. A., Broersen S., Baker C. H., Koot M., van't Wout A. B., Huisman H. G., Miedema F., Tersmette M., Schuitemaker H. Relation of phenotype evolution of HIV-1 to envelope V2 configuration. Science. 1993 Jun 4;260(5113):1513–1516. doi: 10.1126/science.8502996. [DOI] [PubMed] [Google Scholar]
- Hillis D. M., Allard M. W., Miyamoto M. M. Analysis of DNA sequence data: phylogenetic inference. Methods Enzymol. 1993;224:456–487. doi: 10.1016/0076-6879(93)24035-s. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Zhang L. Q., Simmonds P., Ludlam C. A., Brown A. J. Convergent and divergent sequence evolution in the surface envelope glycoprotein of human immunodeficiency virus type 1 within a single infected patient. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4835–4839. doi: 10.1073/pnas.89.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Coffin J. M. Virology. How does variation count? Nature. 1992 Sep 10;359(6391):107–108. doi: 10.1038/359107a0. [DOI] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Jordan C. A., Watkins B. A., Kufta C., Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991 Feb;65(2):736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys B., Karis J., Fadeel B., Valentin A., Norkrans G., Hagberg L., Chiodi F. V3 sequences of paired HIV-1 isolates from blood and cerebrospinal fluid cluster according to host and show variation related to the clinical stage of disease. Virology. 1993 Oct;196(2):475–483. doi: 10.1006/viro.1993.1503. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Korber B., Myers G. Signature pattern analysis: a method for assessing viral sequence relatedness. AIDS Res Hum Retroviruses. 1992 Sep;8(9):1549–1560. doi: 10.1089/aid.1992.8.1549. [DOI] [PubMed] [Google Scholar]
- Korber B., Wolinsky S., Haynes B., Kunstman K., Levy R., Furtado M., Otto P., Myers G. HIV-1 intrapatient sequence diversity in the immunogenic V3 region. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1461–1465. doi: 10.1089/aid.1992.8.1461. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Bergeron L., Dorfman T., Haseltine W., Sodroski J. Attenuation of human immunodeficiency virus type 1 cytopathic effect by a mutation affecting the transmembrane envelope glycoprotein. J Virol. 1991 Jan;65(1):281–291. doi: 10.1128/jvi.65.1.281-291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kuiken C. L., Zwart G., Baan E., Coutinho R. A., van den Hoek J. A., Goudsmit J. Increasing antigenic and genetic diversity of the V3 variable domain of the human immunodeficiency virus envelope protein in the course of the AIDS epidemic. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9061–9065. doi: 10.1073/pnas.90.19.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken C. L., de Jong J. J., Baan E., Keulen W., Tersmette M., Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992 Jul;66(7):4622–4627. doi: 10.1128/jvi.66.7.4622-4627.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi K., Conway B., Cunningham S., Berson A., Evans C., Iversen A. K., Colvin D., Gallo M. V., Coutre S., Shpaer E. G. Human immunodeficiency virus type 1 envelope gene structure and diversity in vivo and after cocultivation in vitro. J Virol. 1992 Feb;66(2):875–885. doi: 10.1128/jvi.66.2.875-885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. R., Syu W. J., Du B., Matsuda M., Tan S., Wolf A., Essex M., Lee T. H. Nonrandom distribution of gp120 N-linked glycosylation sites important for infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2213–2217. doi: 10.1073/pnas.89.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C. K., Spellman M. W., Riddle L., Harris R. J., Thomas J. N., Gregory T. J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990 Jun 25;265(18):10373–10382. [PubMed] [Google Scholar]
- Levy R. M., Russell E., Yungbluth M., Hidvegi D. F., Brody B. A., Dal Canto M. C. The efficacy of image-guided stereotactic brain biopsy in neurologically symptomatic acquired immunodeficiency syndrome patients. Neurosurgery. 1992 Feb;30(2):186–190. doi: 10.1227/00006123-199202000-00006. [DOI] [PubMed] [Google Scholar]
- Li X. L., Moudgil T., Vinters H. V., Ho D. D. CD4-independent, productive infection of a neuronal cell line by human immunodeficiency virus type 1. J Virol. 1990 Mar;64(3):1383–1387. doi: 10.1128/jvi.64.3.1383-1387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kappes J. C., Conway J. A., Price R. W., Shaw G. M., Hahn B. H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991 Aug;65(8):3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L., Rasool N., Kang C. Y. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J Virol. 1993 Jan;67(1):584–588. doi: 10.1128/jvi.67.1.584-588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating J. A., Cordell J., Dean C. J., Balfe P. Synergistic interaction between ligands binding to the CD4 binding site and V3 domain of human immunodeficiency virus type I gp120. Virology. 1992 Dec;191(2):732–742. doi: 10.1016/0042-6822(92)90249-o. [DOI] [PubMed] [Google Scholar]
- McNearney T., Westervelt P., Thielan B. J., Trowbridge D. B., Garcia J., Whittier R., Ratner L. Limited sequence heterogeneity among biologically distinct human immunodeficiency virus type 1 isolates from individuals involved in a clustered infectious outbreak. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1917–1921. doi: 10.1073/pnas.87.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich L., Margolin B., Swanstrom R. V3 loop of the human immunodeficiency virus type 1 Env protein: interpreting sequence variability. J Virol. 1993 Sep;67(9):5623–5634. doi: 10.1128/jvi.67.9.5623-5634.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Nara P. L. The role of the V3 loop of gp120 in HIV infection. AIDS. 1991;5 (Suppl 2):S21–S33. doi: 10.1097/00002030-199101001-00004. [DOI] [PubMed] [Google Scholar]
- Myers G., Lenroot R. HIV glycosylation: what does it portend? AIDS Res Hum Retroviruses. 1992 Aug;8(8):1459–1460. doi: 10.1089/aid.1992.8.1459. [DOI] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Pang S., Vinters H. V., Akashi T., O'Brien W. A., Chen I. S. HIV-1 env sequence variation in brain tissue of patients with AIDS-related neurologic disease. J Acquir Immune Defic Syndr. 1991;4(11):1082–1092. [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tilley S. A. Conformational changes affecting the V3 and CD4-binding domains of human immunodeficiency virus type 1 gp120 associated with env processing and with binding of ligands to these sites. J Virol. 1993 Sep;67(9):5692–5697. doi: 10.1128/jvi.67.9.5692-5697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer L. R. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992 Jan;51(1):3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Sharpless N. E., O'Brien W. A., Verdin E., Kufta C. V., Chen I. S., Dubois-Dalcq M. Human immunodeficiency virus type 1 tropism for brain microglial cells is determined by a region of the env glycoprotein that also controls macrophage tropism. J Virol. 1992 Apr;66(4):2588–2593. doi: 10.1128/jvi.66.4.2588-2593.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell-line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Automatic generation of primary sequence patterns from sets of related protein sequences. Proc Natl Acad Sci U S A. 1990 Jan;87(1):118–122. doi: 10.1073/pnas.87.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Pattern-induced multi-sequence alignment (PIMA) algorithm employing secondary structure-dependent gap penalties for use in comparative protein modelling. Protein Eng. 1992 Jan;5(1):35–41. doi: 10.1093/protein/5.1.35. [DOI] [PubMed] [Google Scholar]
- Stamatatos L., Cheng-Mayer C. Evidence that the structural conformation of envelope gp120 affects human immunodeficiency virus type 1 infectivity, host range, and syncytium-forming ability. J Virol. 1993 Sep;67(9):5635–5639. doi: 10.1128/jvi.67.9.5635-5639.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuler H., Storch-Hagenlocher B., Wildemann B. Distinct populations of human immunodeficiency virus type 1 in blood and cerebrospinal fluid. AIDS Res Hum Retroviruses. 1992 Jan;8(1):53–59. doi: 10.1089/aid.1992.8.53. [DOI] [PubMed] [Google Scholar]
- Tornatore C., Nath A., Amemiya K., Major E. O. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991 Nov;65(11):6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian J. P., Meyerhans A., Asjö B., Wain-Hobson S. Selection, recombination, and G----A hypermutation of human immunodeficiency virus type 1 genomes. J Virol. 1991 Apr;65(4):1779–1788. doi: 10.1128/jvi.65.4.1779-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S. HIV genome variability in vivo. AIDS. 1989;3 (Suppl 1):S13–S18. doi: 10.1097/00002030-198901001-00003. [DOI] [PubMed] [Google Scholar]
- Watkins B. A., Dorn H. H., Kelly W. B., Armstrong R. C., Potts B. J., Michaels F., Kufta C. V., Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990 Aug 3;249(4968):549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- Weber J., Clapham P., McKeating J., Stratton M., Robey E., Weiss R. Infection of brain cells by diverse human immunodeficiency virus isolates: role of CD4 as receptor. J Gen Virol. 1989 Oct;70(Pt 10):2653–2660. doi: 10.1099/0022-1317-70-10-2653. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Trowbridge D. B., Epstein L. G., Blumberg B. M., Li Y., Hahn B. H., Shaw G. M., Price R. W., Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992 Apr;66(4):2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs T. F., Zwart G., Bakker M., Goudsmit J. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology. 1992 Jul;189(1):103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- Wolinsky S. M., Wike C. M., Korber B. T., Hutto C., Parks W. P., Rosenblum L. L., Kunstman K. J., Furtado M. R., Muñoz J. L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992 Feb 28;255(5048):1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Wyatt R., Thali M., Tilley S., Pinter A., Posner M., Ho D., Robinson J., Sodroski J. Relationship of the human immunodeficiency virus type 1 gp120 third variable loop to a component of the CD4 binding site in the fourth conserved region. J Virol. 1992 Dec;66(12):6997–7004. doi: 10.1128/jvi.66.12.6997-7004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Q., MacKenzie P., Cleland A., Holmes E. C., Brown A. J., Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993 Jun;67(6):3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Mo H., Wang N., Nam D. S., Cao Y., Koup R. A., Ho D. D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993 Aug 27;261(5125):1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]




