Abstract
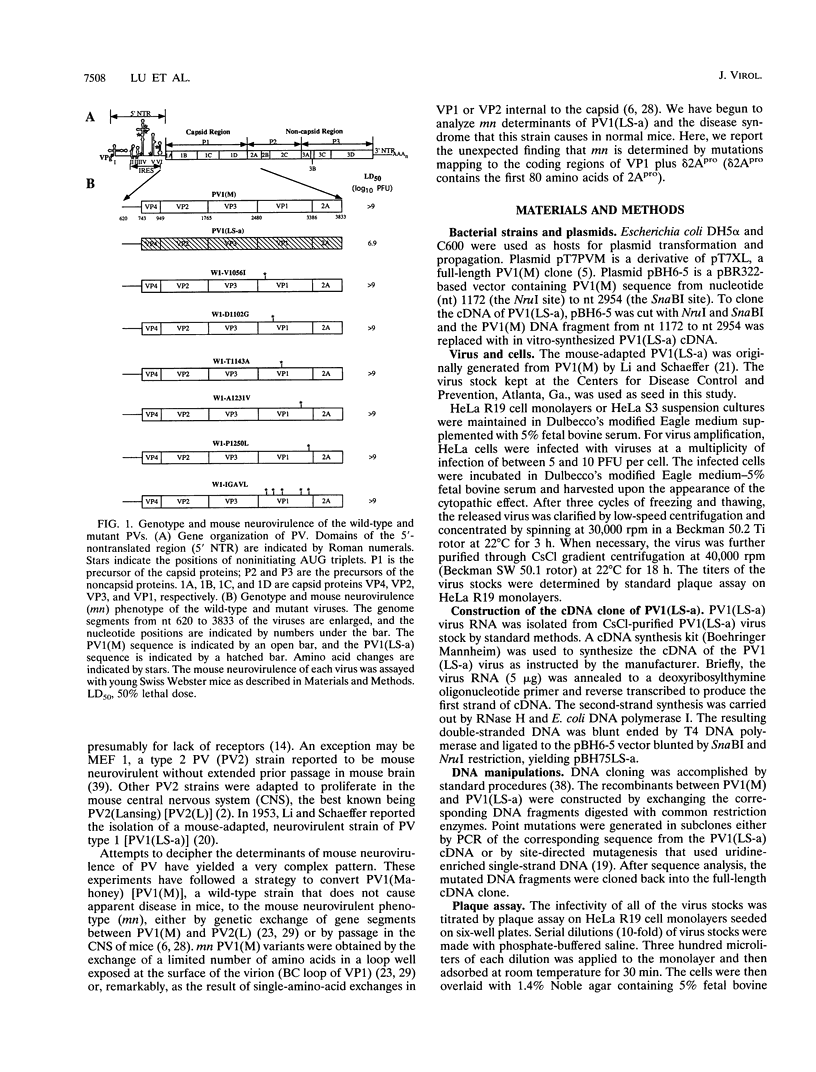
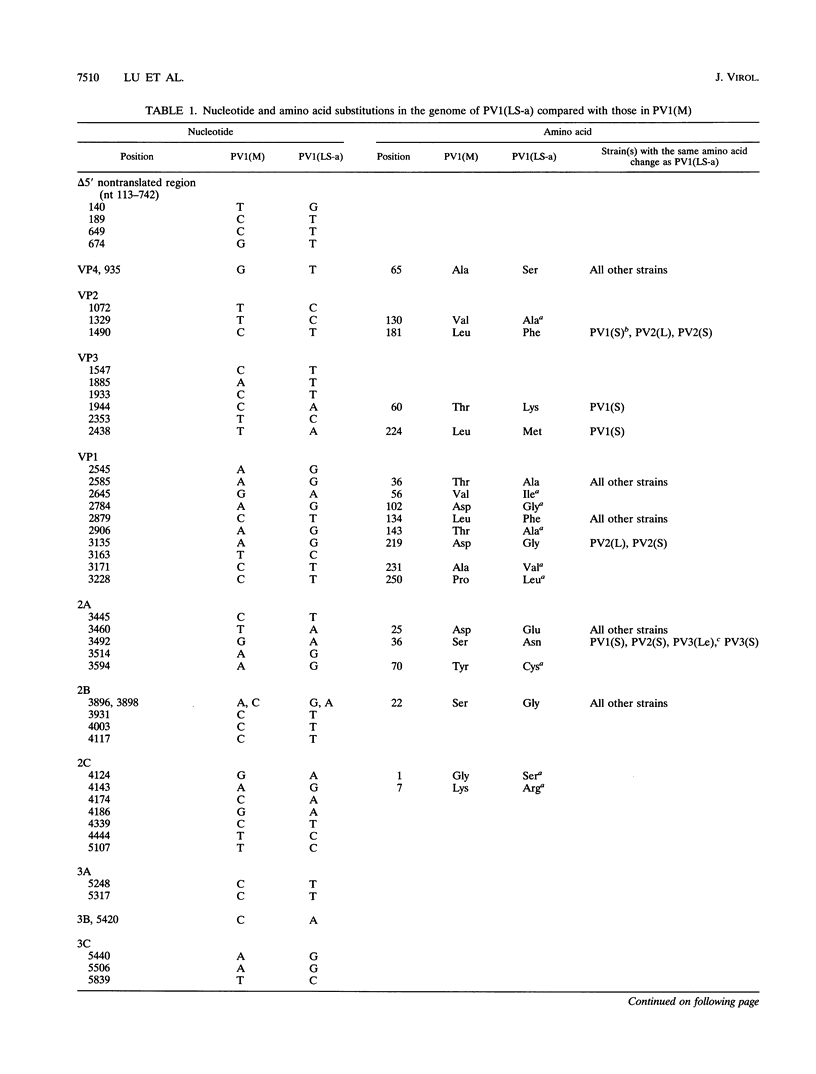
Poliovirus type 1 strain LS-a [PV1(LS-a)] is a OV variant adapted to mice by multiple passages through mouse and monkey tissues. To investigate the molecular basis underlying mouse neurovirulence of PV1(LS-a), a cDNA of the viral genome containing nucleotides 112 to 7441 was cloned, and the nucleotide sequence was determined. Compared with that of the mouse avirulent progenitor PV1(Mahoney), 54 nucleotide changes were found in the genome of the PV1(LS-a) virus, resulting in 20 amino acid substitutions in the virus polyprotein. Whereas the nucleotide changes were scattered throughout the genome, the amino acid substitutions were largely clustered in the capsid proteins and, to a certain extent, in the virus proteinase 2Apro. By in vitro mutagenesis, PV1(LS-a)-specific capsid mutations were introduced into a cDNA clone of PV1(Mahoney). We show that neither the individual amino acid mutations nor combinations of mutations in the region encoding VP1 conferred to PV1(Mahoney) the mouse-adapted phenotype of PV1(LS-a). Chimeric cDNA studies demonstrated that a recombinant type 1 virus containing the PV1(LS-a) sequence from nucleotide 2470 to nucleotide 3625 displayed a neurovirulent phenotype in mice. Further dissection of this region revealed that mouse neurovirulence of PV1(LS-a) was determined by multiple mutations in regions encoding both viral proteinase 2Apro and capsid protein VP1. The mouse neurovirulent viruses, PV1(LS-a), W1-M/LS-Pf [nucleotides 496 to 3625 from PV1(LS-a)], and W1-M/LS-NP [nucleotides 2470 to 3625 from PV1(LS-a)], showed increased sensitivity to heat treatment at 45 degrees C for 1 h. Surprisingly, the thermolabile phenotype was also displayed by a recombinant of PV1(Mahoney) carrying a PV1(LS-a) DNA fragment encoding the N-terminal portion of 2Apro. This suggests that base substitutions in the region encoding 2Apro affected capsid stability, thereby contributing to the neurovirulence of the virus in mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmeyer R., Murdin A. D., Harber J. J., Wimmer E. Construction and characterization of a poliovirus/rhinovirus antigenic hybrid. Virology. 1991 Oct;184(2):636–644. doi: 10.1016/0042-6822(91)90433-c. [DOI] [PubMed] [Google Scholar]
- Bernhardt G., Bibb J. A., Bradley J., Wimmer E. Molecular characterization of the cellular receptor for poliovirus. Virology. 1994 Feb 15;199(1):105–113. doi: 10.1006/viro.1994.1102. [DOI] [PubMed] [Google Scholar]
- Bernhardt G., Harber J., Zibert A., deCrombrugghe M., Wimmer E. The poliovirus receptor: identification of domains and amino acid residues critical for virus binding. Virology. 1994 Sep;203(2):344–356. doi: 10.1006/viro.1994.1493. [DOI] [PubMed] [Google Scholar]
- Cao X., Kuhn R. J., Wimmer E. Replication of poliovirus RNA containing two VPg coding sequences leads to a specific deletion event. J Virol. 1993 Sep;67(9):5572–5578. doi: 10.1128/jvi.67.9.5572-5578.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc T., Hogle J., Le Blay H., Horaud F., Blondel B. Molecular characterization of mouse-virulent poliovirus type 1 Mahoney mutants: involvement of residues of polypeptides VP1 and VP2 located on the inner surface of the capsid protein shell. J Virol. 1993 Jul;67(7):3808–3817. doi: 10.1128/jvi.67.7.3808-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty W. G., Semler B. L. Expression of virus-encoded proteinases: functional and structural similarities with cellular enzymes. Microbiol Rev. 1993 Dec;57(4):781–822. doi: 10.1128/mr.57.4.781-822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- Hambidge S. J., Sarnow P. Translational enhancement of the poliovirus 5' noncoding region mediated by virus-encoded polypeptide 2A. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10272–10276. doi: 10.1073/pnas.89.21.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber J. J., Bradley J., Anderson C. W., Wimmer E. Catalysis of poliovirus VP0 maturation cleavage is not mediated by serine 10 of VP2. J Virol. 1991 Jan;65(1):326–334. doi: 10.1128/jvi.65.1.326-334.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C. U., Fäcke M., Kräusslich H. G., Lee C. K., Wimmer E. Characterization of poliovirus 2A proteinase by mutational analysis: residues required for autocatalytic activity are essential for induction of cleavage of eukaryotic initiation factor 4F polypeptide p220. J Virol. 1991 Aug;65(8):4226–4231. doi: 10.1128/jvi.65.8.4226-4231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Koike S., Horie H., Ise I., Okitsu A., Yoshida M., Iizuka N., Takeuchi K., Takegami T., Nomoto A. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 1990 Oct;9(10):3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Ise I., Sato Y., Yonekawa H., Gotoh O., Nomoto A. A second gene for the African green monkey poliovirus receptor that has no putative N-glycosylation site in the functional N-terminal immunoglobulin-like domain. J Virol. 1992 Dec;66(12):7059–7066. doi: 10.1128/jvi.66.12.7059-7066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Taya C., Kurata T., Abe S., Ise I., Yonekawa H., Nomoto A. Transgenic mice susceptible to poliovirus. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):951–955. doi: 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. P., SCHAEFFER M. Adaptation of type I poliomyelitis virus to mice. Proc Soc Exp Biol Med. 1953 Mar;82(3):477–481. doi: 10.3181/00379727-82-20151. [DOI] [PubMed] [Google Scholar]
- LI C. P., SCHAEFFER M. Isolation of a non-neurotropic variant of type I poliomyelitis virus. Proc Soc Exp Biol Med. 1954 Oct;87(1):148–153. doi: 10.3181/00379727-87-21317. [DOI] [PubMed] [Google Scholar]
- Macadam A. J., Ferguson G., Fleming T., Stone D. M., Almond J. W., Minor P. D. Role for poliovirus protease 2A in cap independent translation. EMBO J. 1994 Feb 15;13(4):924–927. doi: 10.1002/j.1460-2075.1994.tb06336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Wychowski C., Couderc T., Crainic R., Hogle J., Girard M. Engineering a poliovirus type 2 antigenic site on a type 1 capsid results in a chimaeric virus which is neurovirulent for mice. EMBO J. 1988 Sep;7(9):2839–2847. doi: 10.1002/j.1460-2075.1988.tb03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Minor P. D. Antigenic structure of picornaviruses. Curr Top Microbiol Immunol. 1990;161:121–154. doi: 10.1007/978-3-642-75602-3_5. [DOI] [PubMed] [Google Scholar]
- Molla A., Paul A. V., Schmid M., Jang S. K., Wimmer E. Studies on dicistronic polioviruses implicate viral proteinase 2Apro in RNA replication. Virology. 1993 Oct;196(2):739–747. doi: 10.1006/viro.1993.1531. [DOI] [PubMed] [Google Scholar]
- Morrison M. E., Racaniello V. R. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992 May;66(5):2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss E. G., Racaniello V. R. Host range determinants located on the interior of the poliovirus capsid. EMBO J. 1991 May;10(5):1067–1074. doi: 10.1002/j.1460-2075.1991.tb08046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Bradley J., Yang X. F., Wimmer E., Moss E. G., Racaniello V. R. Poliovirus host range is determined by a short amino acid sequence in neutralization antigenic site I. Science. 1988 Jul 8;241(4862):213–215. doi: 10.1126/science.2838906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J. F., Piatti P. G., Gorman B. M., Burrage T. G., Ryan M. D., Flint M., Brown F. Foot-and-mouth disease virus particles contain replicase protein 3D. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):733–737. doi: 10.1073/pnas.91.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Kräusslich H. G., Toyoda H., Dunn J. J., Wimmer E. Poliovirus polypeptide precursors: expression in vitro and processing by exogenous 3C and 2A proteinases. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4002–4006. doi: 10.1073/pnas.84.12.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Oh C. K., Cunningham L. L., Calenoff M., Jubelt B. Localization of genomic regions specific for the attenuated, mouse-adapted poliovirus type 2 strain W-2. J Gen Virol. 1990 Jan;71(Pt 1):43–52. doi: 10.1099/0022-1317-71-1-43. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R. B., Costantini F., Gorgacz E. J., Lee J. J., Racaniello V. R. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell. 1990 Oct 19;63(2):353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem. 1989 Sep 5;264(25):14587–14590. [PubMed] [Google Scholar]
- Schlesinger R. W., Morgan I. M., Olitsky P. K. TRANSMISSION TO RODENTS OF LANSING TYPE POLIOMYELITIS VIRUS ORIGINATING IN THE MIDDLE EAST. Science. 1943 Nov 19;98(2551):452–454. doi: 10.1126/science.98.2551.452. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Poliovirus translation. Curr Top Microbiol Immunol. 1990;161:23–47. doi: 10.1007/978-3-642-75602-3_2. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- Wimmer E., Hellen C. U., Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Jacobson D. H., Martin A., Wychowski C., Girard M., Filman D. J., Hogle J. M. Three-dimensional structure of a mouse-adapted type 2/type 1 poliovirus chimera. EMBO J. 1991 Sep;10(9):2331–2341. doi: 10.1002/j.1460-2075.1991.tb07772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf S., Bradley J., Wimmer E., Studier F. W., Dunn J. J. Synthesis of infectious poliovirus RNA by purified T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]




