Abstract
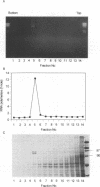
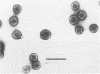
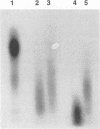
Double-stranded RNA viruses were detected in a strain of Blastomyces dermatitidis isolated from a patient in Uganda. The viral particles are spherical (mostly 44 to 50 nm in diameter) and consist of about 25% double-stranded RNA (5 kb) and 75% protein (90 kDa). The virus contains transcriptional RNA polymerase activity; it synthesized single-stranded RNA in vitro in a conservative manner. The newly synthesized single-stranded RNA was a full-length strand, and the rate of chain elongation was approximately 170 nucleotides per min. The virus-containing strain shows no morphological difference from virus-free strains in the mycelial phase. Although the association with the presence of the virus is unclear, the virus-infected strain converts to the yeast form at 37 degrees C, but the yeast cells fail to multiply at that temperature.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry E. A., Bevan E. A. A new species of double-stranded RNA from yeast. Nature. 1972 Sep 29;239(5370):279–280. doi: 10.1038/239279a0. [DOI] [PubMed] [Google Scholar]
- Borré E., Morgantini L. E., Ortali V., Tonolo A. Production of lytic plaques of viral origin in Penicillium. Nature. 1971 Feb 19;229(5286):568–569. doi: 10.1038/229568b0. [DOI] [PubMed] [Google Scholar]
- Esteban R., Wickner R. B. Three different M1 RNA-containing viruslike particle types in Saccharomyces cerevisiae: in vitro M1 double-stranded RNA synthesis. Mol Cell Biol. 1986 May;6(5):1552–1561. doi: 10.1128/mcb.6.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Esteban R., Esteban L. M., Wickner R. B. Portable encapsidation signal of the L-A double-stranded RNA virus of S. cerevisiae. Cell. 1990 Aug 24;62(4):819–828. doi: 10.1016/0092-8674(90)90125-x. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Esteban R., Wickner R. B. In vitro L-A double-stranded RNA synthesis in virus-like particles from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4433–4437. doi: 10.1073/pnas.83.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Wickner R. B. Interaction of two cis sites with the RNA replicase of the yeast L-A virus. J Biol Chem. 1992 Feb 5;267(4):2708–2713. [PubMed] [Google Scholar]
- Kandel J. S., Stern T. A. Killer phenomenon in pathogenic yeast. Antimicrob Agents Chemother. 1979 Apr;15(4):568–571. doi: 10.1128/aac.15.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., Standard P. G., Weeks R. J., Padhye A. A. Detection of two Blastomyces dermatitidis serotypes by exoantigen analysis. J Clin Microbiol. 1983 Jul;18(1):110–114. doi: 10.1128/jcm.18.1.110-114.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Perick R., Stamberg J., Ben-Shaul Y. Virus-like particles and cytoplasmic inheritance of plaques in a higher fungus. Nat New Biol. 1973 Jan 24;241(108):108–109. doi: 10.1038/newbio241108a0. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J. Genetic analysis on the incompatibility system of Ajellomyces dermatitidis. Sabouraudia. 1971 Nov;9(3):231–238. [PubMed] [Google Scholar]
- Lemke P. A., Nash C. H. Fungal viruses. Bacteriol Rev. 1974 Mar;38(1):29–56. doi: 10.1128/br.38.1.29-56.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbeek E. J., Hermans J. M., Stumm C., Muytjens H. L. High incidence of sensitivity to yeast killer toxins among Candida and Torulopsis isolates of human origin. Antimicrob Agents Chemother. 1980 Mar;17(3):350–354. doi: 10.1128/aac.17.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Fink G. R. A nucleic acid associated with a killer strain of yeast. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1069–1072. doi: 10.1073/pnas.70.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. D., Leibowitz M. J., Wickner R. B. Virion DNA-independent RNA polymerase from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Jun 11;8(11):2349–2363. doi: 10.1093/nar/8.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. A., Bozarth R. F., Adler J., Mackenzie D. W. Proteinaceous virus-like particles from an isolate of Aspergillus flavus. J Virol. 1974 Feb;13(2):532–534. doi: 10.1128/jvi.13.2.532-534.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]