Abstract
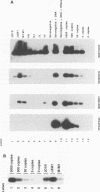
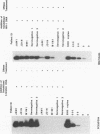
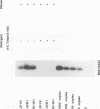
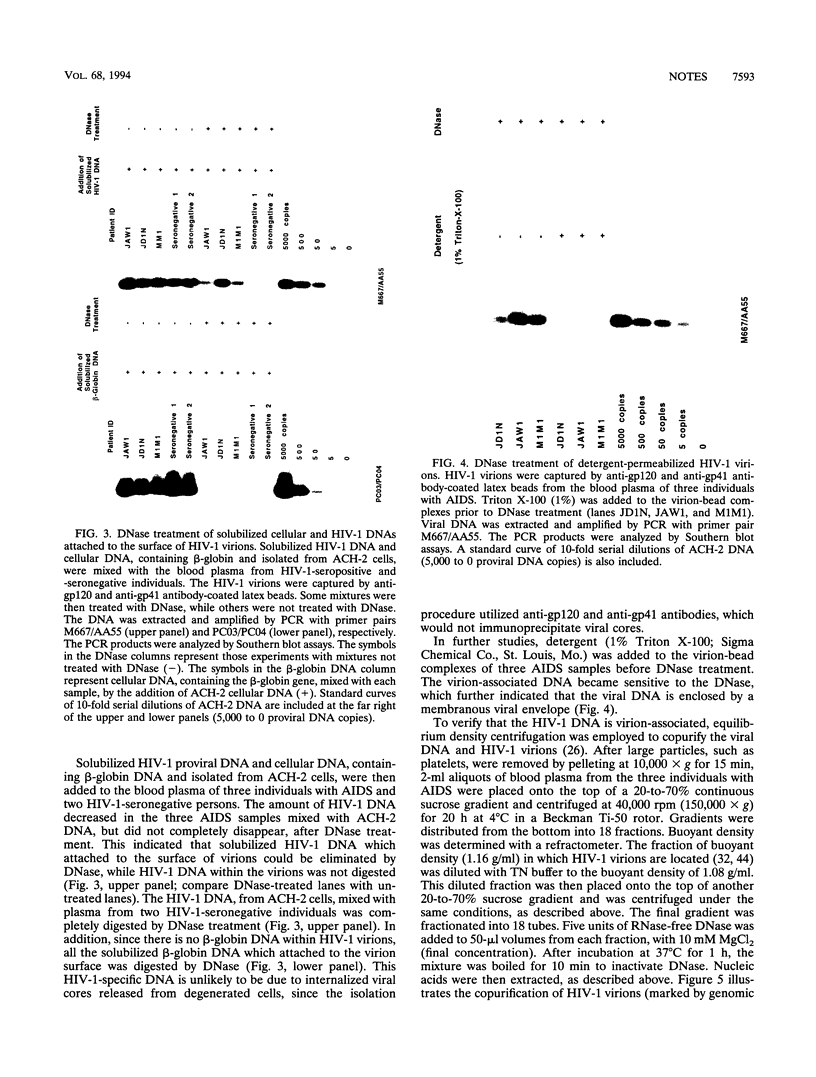
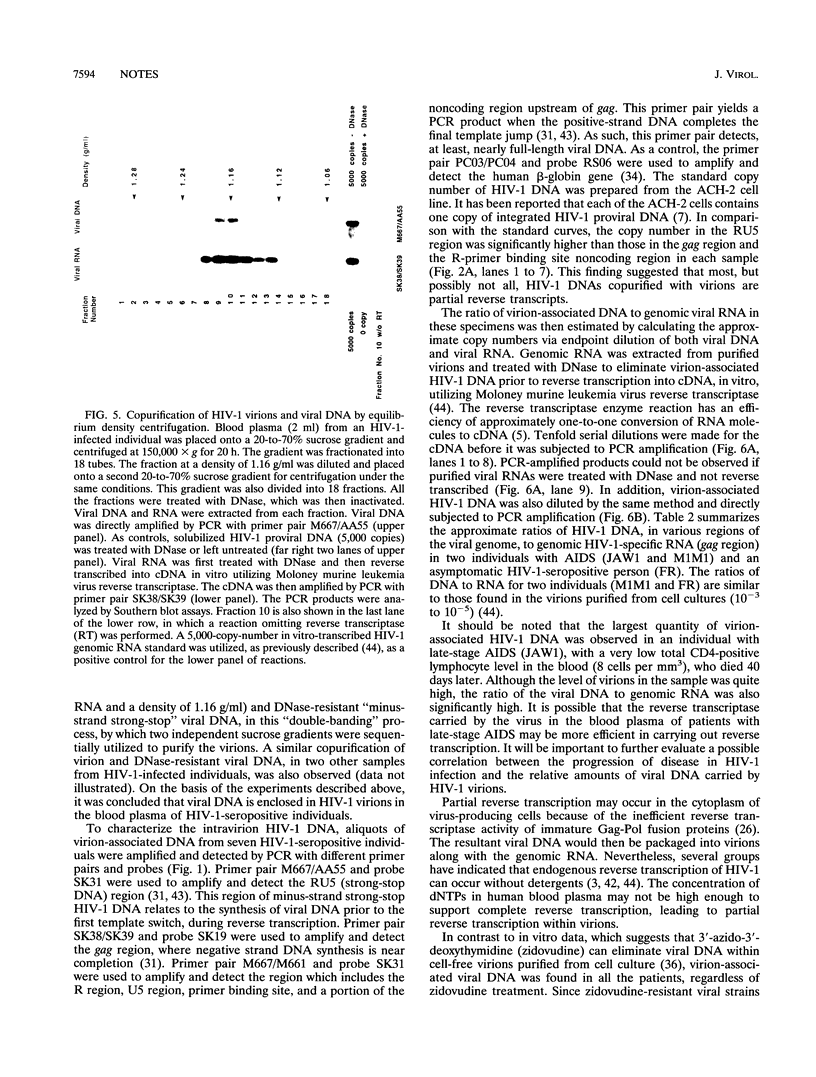
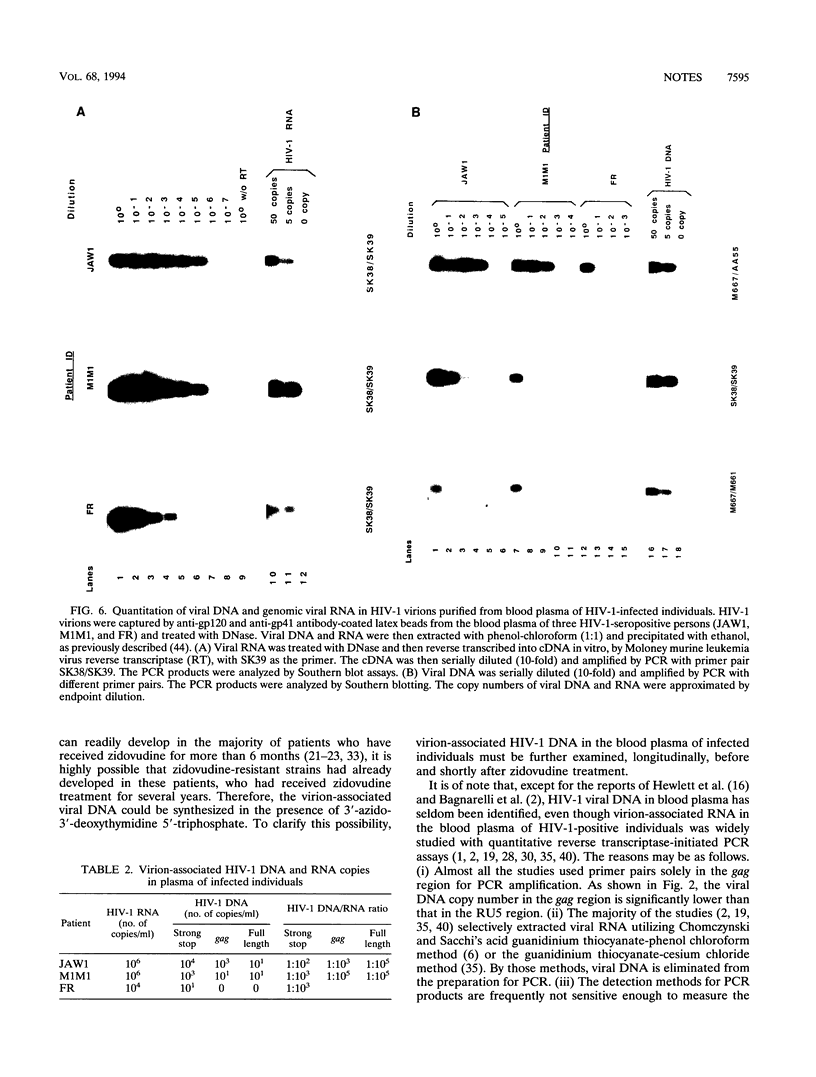
Variable levels of viral DNA have been demonstrated within human immunodeficiency virus type 1 (HIV-1) virions purified from cell cultures. In the present studies, it is demonstrated that DNase-resistant viral DNA is associated with HIV-1 virions purified from the peripheral blood plasma of both symptomatic and asymptomatic HIV-1-infected individuals. The differences in viral DNA copy numbers, detected by quantitative PCR in various regions of the HIV-1 genome, indicated that the intravirion HIV-1 DNA is frequently, but perhaps not totally, the result of partial reverse transcription. These in vivo data suggest that it may be valuable to further investigate the impact of virion-associated viral DNA upon the efficiency of intra- and interhost HIV-1 transmission modes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki-Sei S., Yarchoan R., Kageyama S., Hoekzema D. T., Pluda J. M., Wyvill K. M., Broder S., Mitsuya H. Plasma HIV-1 viremia in HIV-1 infected individuals assessed by polymerase chain reaction. AIDS Res Hum Retroviruses. 1992 Jul;8(7):1263–1270. doi: 10.1089/aid.1992.8.1263. [DOI] [PubMed] [Google Scholar]
- Bagnarelli P., Menzo S., Valenza A., Manzin A., Giacca M., Ancarani F., Scalise G., Varaldo P. E., Clementi M. Molecular profile of human immunodeficiency virus type 1 infection in symptomless patients and in patients with AIDS. J Virol. 1992 Dec;66(12):7328–7335. doi: 10.1128/jvi.66.12.7328-7335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Esoda K., Boone L. R. Equine infectious anemia virus and human immunodeficiency virus DNA synthesis in vitro: characterization of the endogenous reverse transcriptase reaction. J Virol. 1991 Apr;65(4):1952–1959. doi: 10.1128/jvi.65.4.1952-1959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzy M. S., Connell R. S., Kiessling A. A. Detection of human immunodeficiency virus in cell-free seminal fluid. J Acquir Immune Defic Syndr. 1988;1(5):419–424. [PubMed] [Google Scholar]
- Byrne B. C., Li J. J., Sninsky J., Poiesz B. J. Detection of HIV-1 RNA sequences by in vitro DNA amplification. Nucleic Acids Res. 1988 May 11;16(9):4165–4165. doi: 10.1093/nar/16.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- Fujikawa L. S., Salahuddin S. Z., Palestine A. G., Masur H., Nussenblatt R. B., Gallo R. C. Isolation of human T-lymphotropic virus type III from the tears of a patient with the acquired immunodeficiency syndrome. Lancet. 1985 Sep 7;2(8454):529–530. doi: 10.1016/s0140-6736(85)90464-7. [DOI] [PubMed] [Google Scholar]
- Gao W. Y., Cara A., Gallo R. C., Lori F. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8925–8928. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman J. E., Salahuddin S. Z., Sarngadharan M. G., Markham P. D., Gonda M., Sliski A., Gallo R. C. HTLV-III in saliva of people with AIDS-related complex and healthy homosexual men at risk for AIDS. Science. 1984 Oct 26;226(4673):447–449. doi: 10.1126/science.6093247. [DOI] [PubMed] [Google Scholar]
- Henrard D. R., Mehaffey W. F., Allain J. P. A sensitive viral capture assay for detection of plasma viremia in HIV-infected individuals. AIDS Res Hum Retroviruses. 1992 Jan;8(1):47–52. doi: 10.1089/aid.1992.8.47. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Schooley R. T., Rota T. R., Kaplan J. C., Flynn T., Salahuddin S. Z., Gonda M. A., Hirsch M. S. HTLV-III in the semen and blood of a healthy homosexual man. Science. 1984 Oct 26;226(4673):451–453. doi: 10.1126/science.6208608. [DOI] [PubMed] [Google Scholar]
- Holodniy M., Katzenstein D. A., Israelski D. M., Merigan T. C. Reduction in plasma human immunodeficiency virus ribonucleic acid after dideoxynucleoside therapy as determined by the polymerase chain reaction. J Clin Invest. 1991 Nov;88(5):1755–1759. doi: 10.1172/JCI115494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Hénin Y., Mandelbrot L., Henrion R., Pradinaud R., Coulaud J. P., Montagnier L. Virus excretion in the cervicovaginal secretions of pregnant and nonpregnant HIV-infected women. J Acquir Immune Defic Syndr. 1993 Jan;6(1):72–75. [PubMed] [Google Scholar]
- Japour A. J., Chatis P. A., Eigenrauch H. A., Crumpacker C. S. Detection of human immunodeficiency virus type 1 clinical isolates with reduced sensitivity to zidovudine and dideoxyinosine by RNA.RNA hybridization. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3092–3096. doi: 10.1073/pnas.88.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japour A. J., Mayers D. L., Johnson V. A., Kuritzkes D. R., Beckett L. A., Arduino J. M., Lane J., Black R. J., Reichelderfer P. S., D'Aquila R. T. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. The RV-43 Study Group, the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Antimicrob Agents Chemother. 1993 May;37(5):1095–1101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land S., Terloar G., McPhee D., Birch C., Doherty R., Cooper D., Gust I. Decreased in vitro susceptibility to zidovudine of HIV isolates obtained from patients with AIDS. J Infect Dis. 1990 Feb;161(2):326–329. doi: 10.1093/infdis/161.2.326. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Stromberg R. R., Henrard D., Busch M. P. Effect of platelet-associated virus on assays of HIV-1 in plasma. Science. 1993 Dec 3;262(5139):1585–1586. doi: 10.1126/science.8248811. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., Hollander H., Mills J., Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985 Sep 14;2(8455):586–588. [PubMed] [Google Scholar]
- Lori F., di Marzo Veronese F., de Vico A. L., Lusso P., Reitz M. S., Jr, Gallo R. C. Viral DNA carried by human immunodeficiency virus type 1 virions. J Virol. 1992 Aug;66(8):5067–5074. doi: 10.1128/jvi.66.8.5067-5074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Hultgren C., Plikat U., Karlsson A., Wang L., Eriksson S., Wain-Hobson S. Restriction and enhancement of human immunodeficiency virus type 1 replication by modulation of intracellular deoxynucleoside triphosphate pools. J Virol. 1994 Jan;68(1):535–540. doi: 10.1128/jvi.68.1.535-540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J., McKinney N., Christopherson C., Sninsky J., Greenfield L., Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol. 1994 Feb;32(2):292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. A., Namazi A., Kalhor H., Mao S. H., Zack J. A., Chen I. S. Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J Virol. 1994 Feb;68(2):1258–1263. doi: 10.1128/jvi.68.2.1258-1263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Grimes J. M., Lagakos S. W. Effect of stage of disease and drug dose on zidovudine susceptibilities of isolates of human immunodeficiency virus. J Acquir Immune Defic Syndr. 1990;3(8):743–746. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Scadden D. T., Wang Z., Groopman J. E. Quantitation of plasma human immunodeficiency virus type 1 RNA by competitive polymerase chain reaction. J Infect Dis. 1992 Jun;165(6):1119–1123. doi: 10.1093/infdis/165.6.1119. [DOI] [PubMed] [Google Scholar]
- Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992 Aug;66(8):4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Perre P., Simonon A., Msellati P., Hitimana D. G., Vaira D., Bazubagira A., Van Goethem C., Stevens A. M., Karita E., Sondag-Thull D. Postnatal transmission of human immunodeficiency virus type 1 from mother to infant. A prospective cohort study in Kigali, Rwanda. N Engl J Med. 1991 Aug 29;325(9):593–598. doi: 10.1056/NEJM199108293250901. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Vogt M. W., Witt D. J., Craven D. E., Byington R., Crawford D. F., Schooley R. T., Hirsch M. S. Isolation of HTLV-III/LAV from cervical secretions of women at risk for AIDS. Lancet. 1986 Mar 8;1(8480):525–527. doi: 10.1016/s0140-6736(86)90884-6. [DOI] [PubMed] [Google Scholar]
- Winters M. A., Tan L. B., Katzenstein D. A., Merigan T. C. Biological variation and quality control of plasma human immunodeficiency virus type 1 RNA quantitation by reverse transcriptase polymerase chain reaction. J Clin Microbiol. 1993 Nov;31(11):2960–2966. doi: 10.1128/jcm.31.11.2960-2966.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Li S., Perman J., Viscidi R. Persistent diarrhea and fecal shedding of retroviral nucleic acids in children infected with human immunodeficiency virus. J Infect Dis. 1991 Jul;164(1):61–66. doi: 10.1093/infdis/164.1.64. [DOI] [PubMed] [Google Scholar]
- Yong W. H., Wyman S., Levy J. A. Optimal conditions for synthesizing complementary DNA in the HIV-1 endogenous reverse transcriptase reaction. AIDS. 1990 Mar;4(3):199–206. doi: 10.1097/00002030-199003000-00004. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang Y., Spicer T. P., Abbott L. Z., Abbott M., Poiesz B. J. Reverse transcription takes place within extracellular HIV-1 virions: potential biological significance. AIDS Res Hum Retroviruses. 1993 Dec;9(12):1287–1296. doi: 10.1089/aid.1993.9.1287. [DOI] [PubMed] [Google Scholar]