Abstract
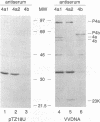
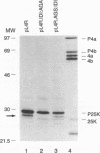
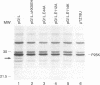
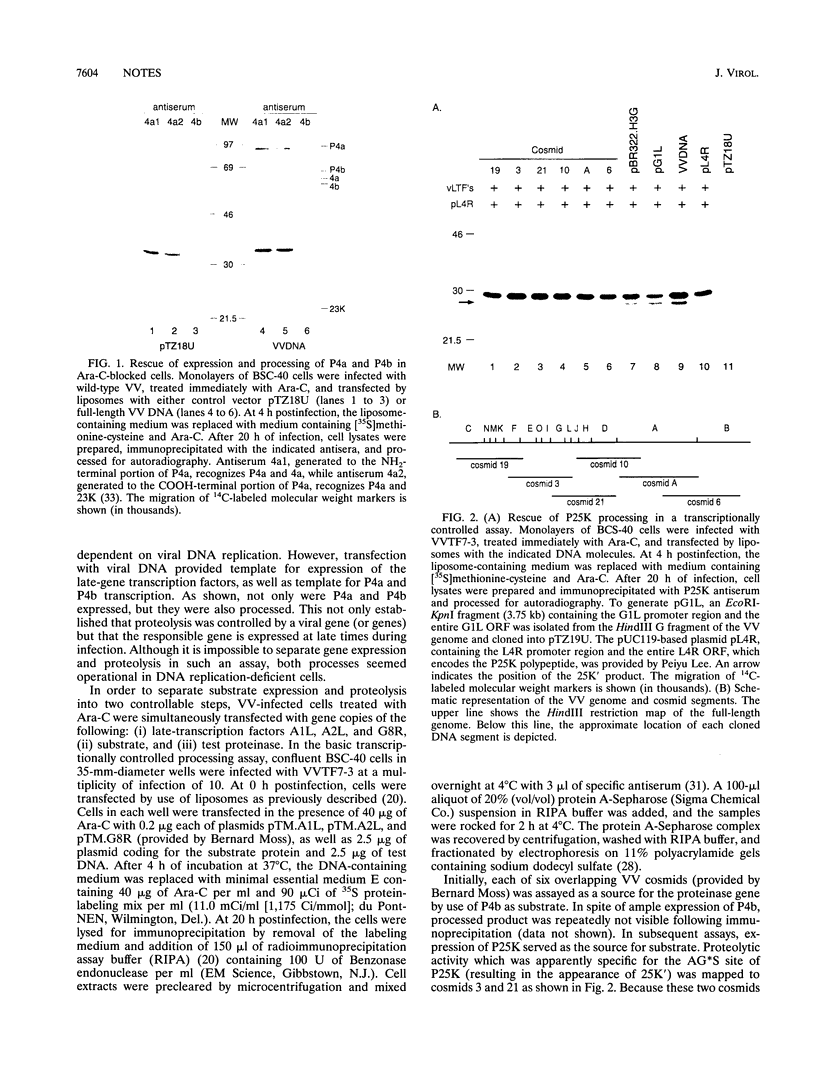
Vaccinia virus maturation into infectious particles appears to be dependent on the proteolytic processing of at least five viral proteins, each containing a conserved AG*X cleavage motif and each requiring proper association with the previrion particle. To identify the responsible proteinase, a transcriptionally controlled trans-processing assay was developed to monitor cleavage at the permissive AG*S site of the P25K core protein precursor. This assay led to the putative identification of a VV proteinase encoded by open reading frame G1L. The predicted protein contains an HXXEH sequence which is a direct inversion of the active site consensus sequence present in thermolysin and other metalloendopeptidases. Site-directed mutation of this consensus sequence suggests that the G1L protein may be a novel, virus-encoded metalloendoproteinase, although confirmation of this activity must await the development of a suitable cell-free processing assay.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affholter J. A., Fried V. A., Roth R. A. Human insulin-degrading enzyme shares structural and functional homologies with E. coli protease III. Science. 1988 Dec 9;242(4884):1415–1418. doi: 10.1126/science.3059494. [DOI] [PubMed] [Google Scholar]
- Arzoglou P., Drillien R., Kirn A. Evidence for an alkaline protease in vaccinia virus. Virology. 1979 May;95(1):211–214. doi: 10.1016/0042-6822(79)90416-1. [DOI] [PubMed] [Google Scholar]
- Becker A. B., Roth R. A. An unusual active site identified in a family of zinc metalloendopeptidases. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3835–3839. doi: 10.1073/pnas.89.9.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. In vitro translation of immediate early, early, and late classes of RNA from vaccinia virus-infected cells. Virology. 1979 Jul 30;96(2):368–380. doi: 10.1016/0042-6822(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Dougherty W. G., Semler B. L. Expression of virus-encoded proteinases: functional and structural similarities with cellular enzymes. Microbiol Rev. 1993 Dec;57(4):781–822. doi: 10.1128/mr.57.4.781-822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Wilson R. E., Brown K., Hickson I. D., Emmerson P. T. Complete nucleotide sequence of the Escherichia coli ptr gene encoding protease III. Nucleic Acids Res. 1986 Oct 10;14(19):7695–7703. doi: 10.1093/nar/14.19.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Hijikata M., Mizushima H., Akagi T., Mori S., Kakiuchi N., Kato N., Tanaka T., Kimura K., Shimotohno K. Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J Virol. 1993 Aug;67(8):4665–4675. doi: 10.1128/jvi.67.8.4665-4675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck J. G., Baldick C. J., Jr, Moss B. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late trans-activator genes. Cell. 1990 Jun 1;61(5):801–809. doi: 10.1016/0092-8674(90)90190-p. [DOI] [PubMed] [Google Scholar]
- Keck J. G., Feigenbaum F., Moss B. Mutational analysis of a predicted zinc-binding motif in the 26-kilodalton protein encoded by the vaccinia virus A2L gene: correlation of zinc binding with late transcriptional transactivation activity. J Virol. 1993 Oct;67(10):5749–5753. doi: 10.1128/jvi.67.10.5749-5753.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Kubo M., Imanaka T. Cloning and nucleotide sequence of the highly thermostable neutral protease gene from Bacillus stearothermophilus. J Gen Microbiol. 1988 Jul;134(7):1883–1892. doi: 10.1099/00221287-134-7-1883. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo W. L., Gehm B. D., Rosner M. R. Cloning and expression of the cDNA for a Drosophila insulin-degrading enzyme. Mol Endocrinol. 1990 Oct;4(10):1580–1591. doi: 10.1210/mend-4-10-1580. [DOI] [PubMed] [Google Scholar]
- Lee P., Hruby D. E. trans processing of vaccinia virus core proteins. J Virol. 1993 Jul;67(7):4252–4263. doi: 10.1128/jvi.67.7.4252-4263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., McGrath W. J., Toledo D. L., Anderson C. W. Viral DNA and a viral peptide can act as cofactors of adenovirus virion proteinase activity. Nature. 1993 Jan 21;361(6409):274–275. doi: 10.1038/361274a0. [DOI] [PubMed] [Google Scholar]
- Massung R. F., Esposito J. J., Liu L. I., Qi J., Utterback T. R., Knight J. C., Aubin L., Yuran T. E., Parsons J. M., Loparev V. N. Potential virulence determinants in terminal regions of variola smallpox virus genome. Nature. 1993 Dec 23;366(6457):748–751. doi: 10.1038/366748a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Letter: Protein cleavage and poxvirus morphogenesis: tryptic peptide analysis of core precursors accumulated by blocking assembly with rifampicin. J Mol Biol. 1973 Dec 5;81(2):267–269. doi: 10.1016/0022-2836(73)90195-2. [DOI] [PubMed] [Google Scholar]
- Shchelkunov S. N., Resenchuk S. M., Totmenin A. V., Blinov V. M., Marennikova S. S., Sandakhchiev L. S. Comparison of the genetic maps of variola and vaccinia viruses. FEBS Lett. 1993 Aug 2;327(3):321–324. doi: 10.1016/0014-5793(93)81013-p. [DOI] [PubMed] [Google Scholar]
- Silver M., Dales S. Biogenesis of vaccina: interrelationship between post-translational cleavage, virus assembly, and maturation. Virology. 1982 Mar;117(2):341–356. doi: 10.1016/0042-6822(82)90474-3. [DOI] [PubMed] [Google Scholar]
- Stern W., Pogo B. G., Dales S. Biogenesis of poxviruses: analysis of the morphogenetic sequence using a conditional lethal mutant defective in envelope self-assembly. Proc Natl Acad Sci U S A. 1977 May;74(5):2162–2166. doi: 10.1073/pnas.74.5.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- VanSlyke J. K., Franke C. A., Hruby D. E. Proteolytic maturation of vaccinia virus core proteins: identification of a conserved motif at the N termini of the 4b and 25K virion proteins. J Gen Virol. 1991 Feb;72(Pt 2):411–416. doi: 10.1099/0022-1317-72-2-411. [DOI] [PubMed] [Google Scholar]
- Vanslyke J. K., Hruby D. E. Immunolocalization of vaccinia virus structural proteins during virion formation. Virology. 1994 Feb;198(2):624–635. doi: 10.1006/viro.1994.1074. [DOI] [PubMed] [Google Scholar]
- Vanslyke J. K., Lee P., Wilson E. M., Hruby D. E. Isolation and analysis of vaccinia virus previrions. Virus Genes. 1993 Dec;7(4):311–324. doi: 10.1007/BF01703388. [DOI] [PubMed] [Google Scholar]
- Vanslyke J. K., Whitehead S. S., Wilson E. M., Hruby D. E. The multistep proteolytic maturation pathway utilized by vaccinia virus P4a protein: a degenerate conserved cleavage motif within core proteins. Virology. 1991 Aug;183(2):467–478. doi: 10.1016/0042-6822(91)90976-i. [DOI] [PubMed] [Google Scholar]
- Whitehead S. S., Hruby D. E. Differential utilization of a conserved motif for the proteolytic maturation of vaccinia virus proteins. Virology. 1994 Apr;200(1):154–161. doi: 10.1006/viro.1994.1174. [DOI] [PubMed] [Google Scholar]