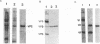Abstract
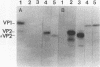
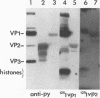
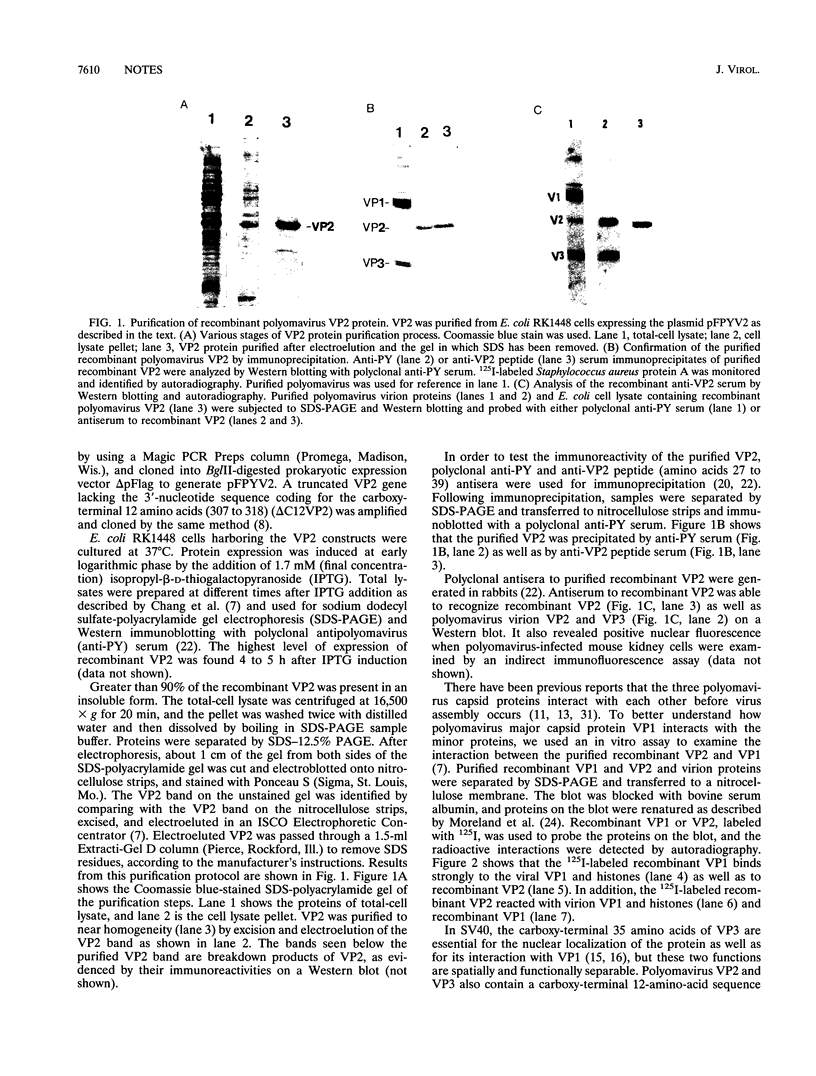
Recombinant polyomavirus VP2 protein was expressed in Escherichia coli (RK1448), using the recombinant expression system pFPYV2. Recombinant VP2 was purified to near homogeneity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroelution, and Extracti-Gel chromatography. Polyclonal serum to this protein which reacted specifically with recombinant VP2 as well as polyomavirus virion VP2 and VP3 on Western blots (immunoblots) was produced. Purified VP2 was used to establish an in vitro protein-protein interaction assay with polyomavirus structural proteins and purified recombinant VP1. Recombinant VP2 interacted with recombinant VP1, virion VP1, and the four virion histones. Recombinant VP1 coimmunoprecipitated with recombinant VP2 or truncated VP2 (delta C12VP2), which lacked the carboxy-terminal 12 amino acids. These experiments confirmed the interaction between VP1 and VP2 and revealed that the carboxyterminal 12 amino acids of VP2 and VP3 were not necessary for formation of this interaction. In vivo VP1-VP2 interaction study accomplished by cotransfection of COS-7 cells with VP2 and truncated VP1 (delta N11VP1) lacking the nuclear localization signal demonstrated that VP2 was capable of translocating delta N11VP1 into the nucleus. These studies suggest that complexes of VP1 and VP2 may be formed in the cytoplasm and cotransported to the nucleus for virion assembly to occur.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barouch D. H., Harrison S. C. Interactions among the major and minor coat proteins of polyomavirus. J Virol. 1994 Jun;68(6):3982–3989. doi: 10.1128/jvi.68.6.3982-3989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H. A., Yguerabide J., Taylor P. Flexibility of the molecular forms of acetylcholinesterase measured with steady-state and time-correlated fluorescence polarization spectroscopy. Biochemistry. 1985 Dec 3;24(25):7140–7147. doi: 10.1021/bi00346a018. [DOI] [PubMed] [Google Scholar]
- Brady J. N., Kendall J. D., Consigli R. A. In vitro reassembly of infectious polyoma virions. J Virol. 1979 Nov;32(2):640–647. doi: 10.1128/jvi.32.2.640-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N'-tetraacetic acid and dithiothreitol. J Virol. 1978 Jul;27(1):193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Cai X., Consigli R. A. Characterization of the DNA binding properties of polyomavirus capsid protein. J Virol. 1993 Oct;67(10):6327–6331. doi: 10.1128/jvi.67.10.6327-6331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Haynes J. I., 2nd, Brady J. N., Consigli R. A. Identification of a nuclear localization sequence in the polyomavirus capsid protein VP2. Virology. 1992 Dec;191(2):978–983. doi: 10.1016/0042-6822(92)90276-u. [DOI] [PubMed] [Google Scholar]
- Chang D., Haynes J. I., 2nd, Brady J. N., Consigli R. A. The use of additive and subtractive approaches to examine the nuclear localization sequence of the polyomavirus major capsid protein VP1. Virology. 1992 Aug;189(2):821–827. doi: 10.1016/0042-6822(92)90615-v. [DOI] [PubMed] [Google Scholar]
- Clever J., Dean D. A., Kasamatsu H. Identification of a DNA binding domain in simian virus 40 capsid proteins Vp2 and Vp3. J Biol Chem. 1993 Oct 5;268(28):20877–20883. [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delos S. E., Montross L., Moreland R. B., Garcea R. L. Expression of the polyomavirus VP2 and VP3 proteins in insect cells: coexpression with the major capsid protein VP1 alters VP2/VP3 subcellular localization. Virology. 1993 May;194(1):393–398. doi: 10.1006/viro.1993.1274. [DOI] [PubMed] [Google Scholar]
- Fattaey A. R., Consigli R. A. Synthesis, posttranslational modifications, and nuclear transport of polyomavirus major capsid protein VP1. J Virol. 1989 Jul;63(7):3168–3175. doi: 10.1128/jvi.63.7.3168-3175.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstová J., Krauzewicz N., Wallace S., Street A. J., Dilworth S. M., Beard S., Griffin B. E. Cooperation of structural proteins during late events in the life cycle of polyomavirus. J Virol. 1993 Mar;67(3):1405–1413. doi: 10.1128/jvi.67.3.1405-1413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea R. L., Benjamin T. L. Isolation and characterization of polyoma nucleoprotein complexes. Virology. 1983 Oct 15;130(1):65–75. doi: 10.1016/0042-6822(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Gharakhanian E., Kasamatsu H. Two independent signals, a nuclear localization signal and a Vp1-interactive signal, reside within the carboxy-35 amino acids of SV40 Vp3. Virology. 1990 Sep;178(1):62–71. doi: 10.1016/0042-6822(90)90379-6. [DOI] [PubMed] [Google Scholar]
- Gharakhanian E., Takahashi J., Clever J., Kasamatsu H. In vitro assay for protein-protein interaction: carboxyl-terminal 40 residues of simian virus 40 structural protein VP3 contain a determinant for interaction with VP1. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6607–6611. doi: 10.1073/pnas.85.18.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. P., Griffith D. L., Rayment I., Murakami W. T., Caspar D. L. Inside polyomavirus at 25-A resolution. Nature. 1992 Feb 13;355(6361):652–654. doi: 10.1038/355652a0. [DOI] [PubMed] [Google Scholar]
- Haynes J. I., 2nd, Chang D., Consigli R. A. Mutations in the putative calcium-binding domain of polyomavirus VP1 affect capsid assembly. J Virol. 1993 May;67(5):2486–2495. doi: 10.1128/jvi.67.5.2486-2495.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzewicz N., Streuli C. H., Stuart-Smith N., Jones M. D., Wallace S., Griffin B. E. Myristylated polyomavirus VP2: role in the life cycle of the virus. J Virol. 1990 Sep;64(9):4414–4420. doi: 10.1128/jvi.64.9.4414-4420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott S. J., Consigli R. A. Production and characterization of monoclonal antibodies to polyomavirus major capsid protein VP1. J Virol. 1985 Nov;56(2):365–372. doi: 10.1128/jvi.56.2.365-372.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Characterization of polyoma DNA-protein complexes. I. Electrophoretic identification of the proteins in a nucleoprotein complex isolated from polyoma-infected cells. J Virol. 1974 Dec;14(6):1326–1336. doi: 10.1128/jvi.14.6.1326-1336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Immunological reactivity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1977 Mar;21(3):1113–1120. doi: 10.1128/jvi.21.3.1113-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Garcea R. L. Characterization of a nuclear localization sequence in the polyomavirus capsid protein VP1. Virology. 1991 Nov;185(1):513–518. doi: 10.1016/0042-6822(91)90811-o. [DOI] [PubMed] [Google Scholar]
- Moreland R. B., Montross L., Garcea R. L. Characterization of the DNA-binding properties of the polyomavirus capsid protein VP1. J Virol. 1991 Mar;65(3):1168–1176. doi: 10.1128/jvi.65.3.1168-1176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necessary P. C., Humphrey P. A., Mahajan P. B., Ebner K. E. Purification of rabbit mammary prolactin receptor by acidic elution from a prolactin affinity column. J Biol Chem. 1984 Jun 10;259(11):6942–6946. [PubMed] [Google Scholar]
- Rayment I., Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982 Jan 14;295(5845):110–115. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli R., Freund R., Dubensky T., Garcea R., Bronson R., Benjamin T. Defect in entry and altered pathogenicity of a polyoma virus mutant blocked in VP2 myristylation. Virology. 1993 Jan;192(1):142–153. doi: 10.1006/viro.1993.1016. [DOI] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986 Sep 12;46(6):895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Müller H., Schmidt M. F., Rott R. Myristoylation of budgerigar fledgling disease virus capsid protein VP2. J Virol. 1989 Jan;63(1):429–431. doi: 10.1128/jvi.63.1.429-431.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi T. DNA-binding properties of the major structural protein of simian virus 40. J Virol. 1986 Sep;59(3):740–742. doi: 10.1128/jvi.59.3.740-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatos N. M., Chakrabarti S., Moss B., Hare J. D. Expression of polyomavirus virion proteins by a vaccinia virus vector: association of VP1 and VP2 with the nuclear framework. J Virol. 1987 Feb;61(2):516–525. doi: 10.1128/jvi.61.2.516-525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli C. H., Griffin B. E. Myristic acid is coupled to a structural protein of polyoma virus and SV40. Nature. 1987 Apr 9;326(6113):619–622. doi: 10.1038/326619a0. [DOI] [PubMed] [Google Scholar]
- Yuen L. K., Consigli R. A. Identification and protein analysis of polyomavirus assembly intermediates from infected primary mouse embryo cells. Virology. 1985 Jul 15;144(1):127–138. doi: 10.1016/0042-6822(85)90311-3. [DOI] [PubMed] [Google Scholar]
- Yuen L. K., Consigli R. A. Improved infectivity of reassembled polyoma virus. J Virol. 1982 Jul;43(1):337–341. doi: 10.1128/jvi.43.1.337-341.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]