Abstract
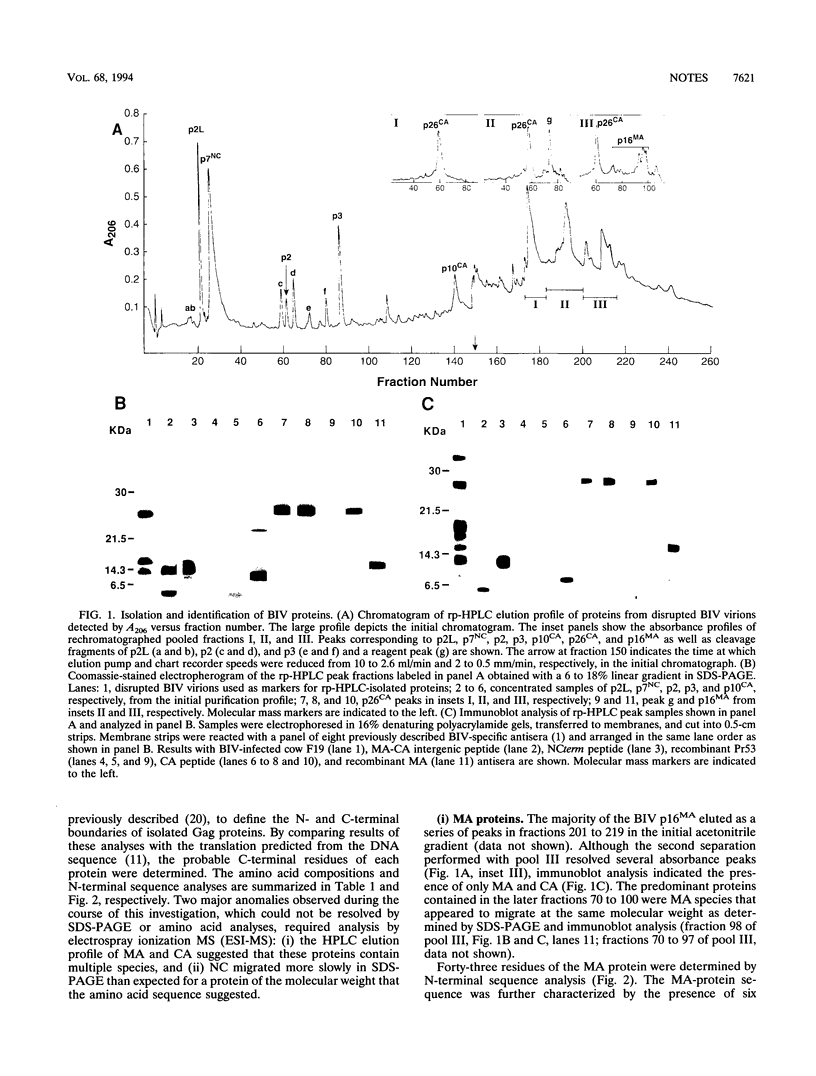
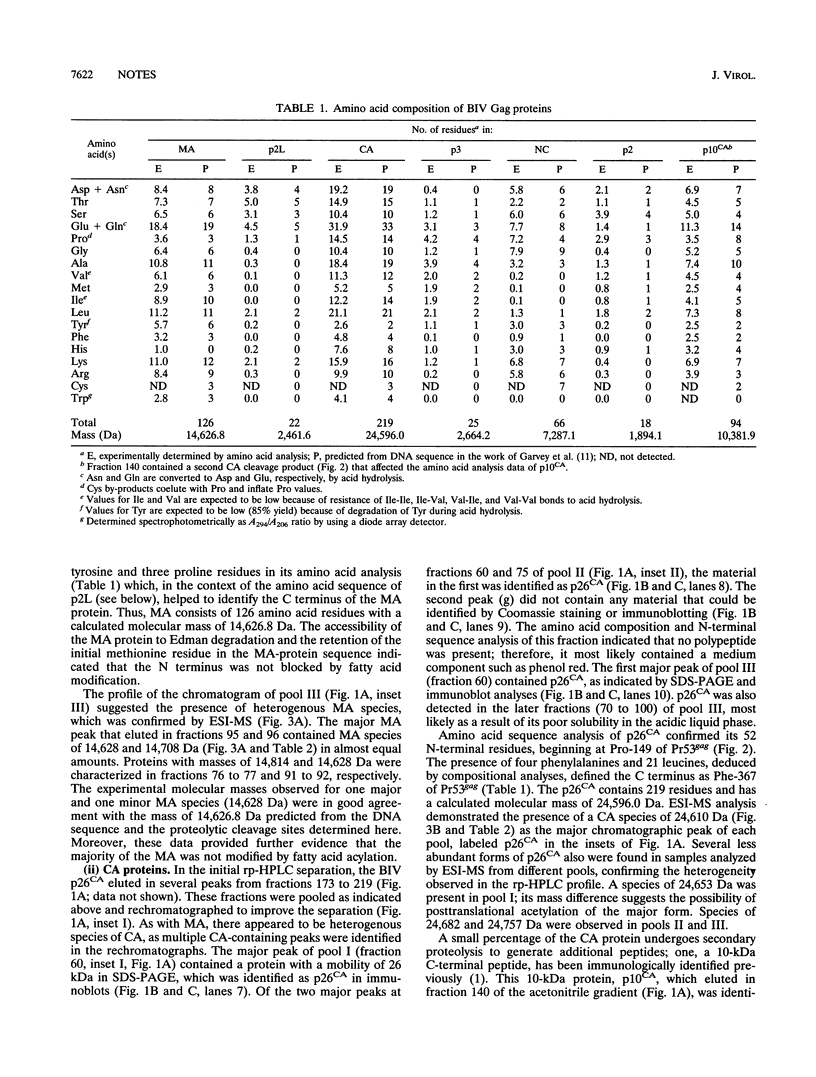
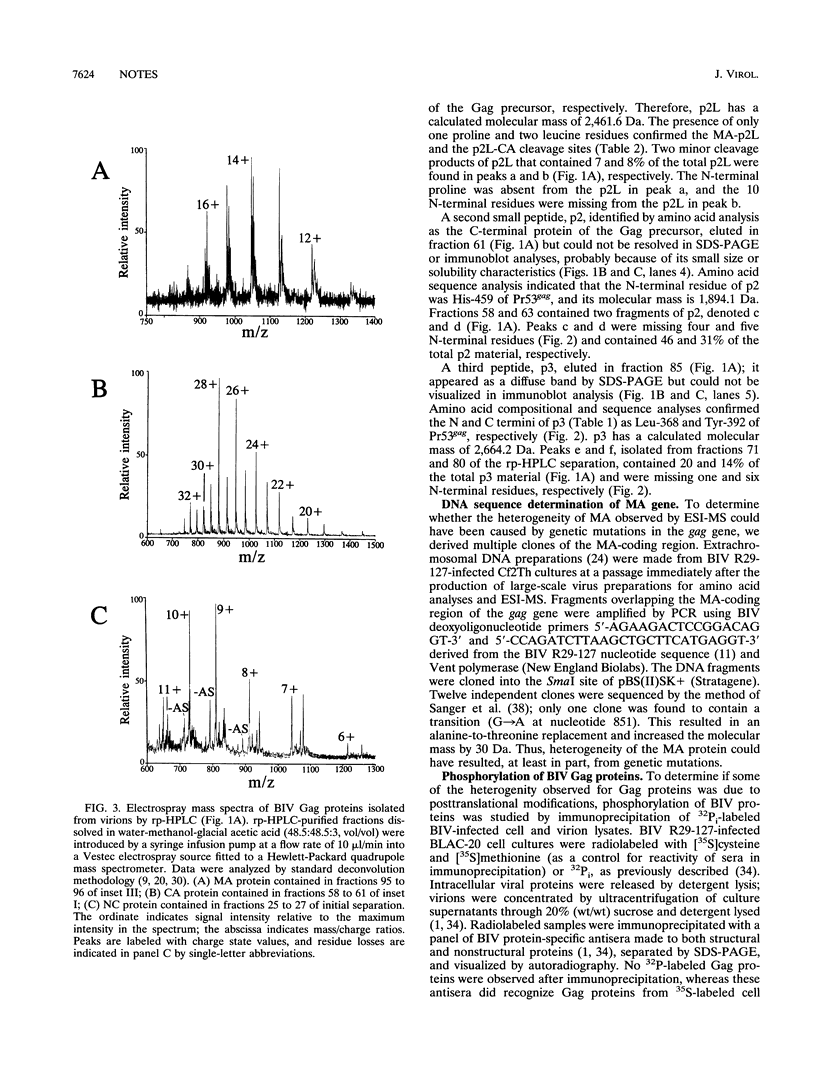
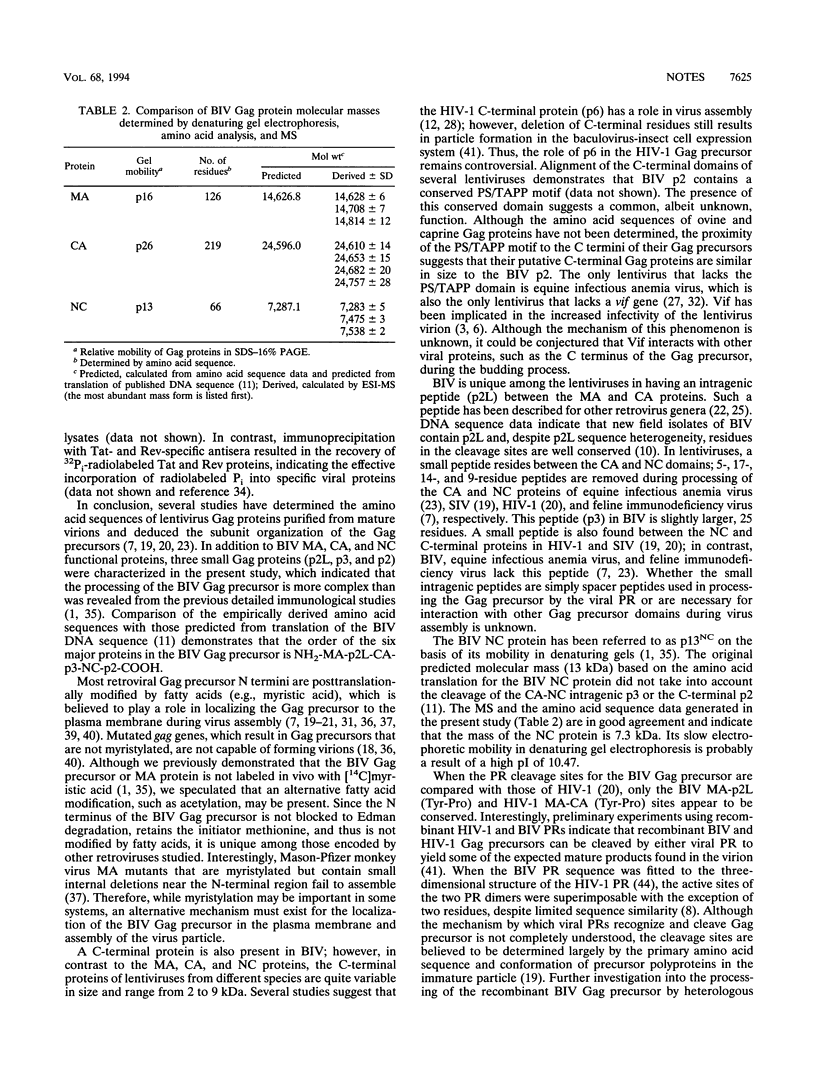
Bovine immunodeficiency virus Gag proteins were purified from virions, and their amino acid sequences and molecular masses were determined. The matrix, capsid, and nucleocapsid (MA, CA, and NC, respectively) and three smaller proteins (p2L, p3, and p2) were found to have molecular masses of 14.6, 24.6, and 7.3 and 2.5, 2.7, and 1.9 kDa, respectively. The order of these six proteins in the Gag precursor, Pr53gag, is NH2-MA-p2L-CA-p3-NC-p2-COOH. In contrast to other retroviral MA proteins, the bovine immunodeficiency virus MA retains its N-terminal methionine and is not modified by fatty acids. In addition, the bovine immunodeficiency virus NC migrates as a 13-kDa protein in denaturing gel electrophoresis; however, its molecular mass was determined to be 7.3 kDa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battles J. K., Hu M. Y., Rasmussen L., Tobin G. J., Gonda M. A. Immunological characterization of the gag gene products of bovine immunodeficiency virus. J Virol. 1992 Dec;66(12):6868–6877. doi: 10.1128/jvi.66.12.6868-6877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton C. V., Hodge H. M., Fine D. L. Comparative large-scale propagation of retroviruses from Old World (Mason-Pfizer monkey virus) and New World (squirrel monkey virus) primates. In Vitro. 1978 Feb;14(2):192–199. doi: 10.1007/BF02618222. [DOI] [PubMed] [Google Scholar]
- Blanc D., Patience C., Schulz T. F., Weiss R., Spire B. Transcomplementation of VIF- HIV-1 mutants in CEM cells suggests that VIF affects late steps of the viral life cycle. Virology. 1993 Mar;193(1):186–192. doi: 10.1006/viro.1993.1114. [DOI] [PubMed] [Google Scholar]
- Braun M. J., Lahn S., Boyd A. L., Kost T. A., Nagashima K., Gonda M. A. Molecular cloning of biologically active proviruses of bovine immunodeficiency-like virus. Virology. 1988 Dec;167(2):515–523. [PubMed] [Google Scholar]
- Carpenter S., Miller L. D., Alexandersen S., Whetstone C. A., VanDerMaaten M. J., Viuff B., Wannemuehler Y., Miller J. M., Roth J. A. Characterization of early pathogenic effects after experimental infection of calves with bovine immunodeficiency-like virus. J Virol. 1992 Feb;66(2):1074–1083. doi: 10.1128/jvi.66.2.1074-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Greene W. C. Functions of the auxiliary gene products of the human immunodeficiency virus type 1. Virology. 1990 Sep;178(1):1–5. doi: 10.1016/0042-6822(90)90373-y. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Schnölzer M., Hasselkus-Light C. S., Henson M., Lerner D. A., Phillips T. R., Wagaman P. C., Kent S. B. Identification of proteolytic processing sites within the Gag and Pol polyproteins of feline immunodeficiency virus. J Virol. 1993 Apr;67(4):1869–1876. doi: 10.1128/jvi.67.4.1869-1876.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenselau C. Beyond gene sequencing: analysis of protein structure with mass spectrometry. Annu Rev Biophys Biophys Chem. 1991;20:205–220. doi: 10.1146/annurev.bb.20.060191.001225. [DOI] [PubMed] [Google Scholar]
- Garvey K. J., Oberste M. S., Elser J. E., Braun M. J., Gonda M. A. Nucleotide sequence and genome organization of biologically active proviruses of the bovine immunodeficiency-like virus. Virology. 1990 Apr;175(2):391–409. doi: 10.1016/0042-6822(90)90424-p. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Ozel M., Pauli G. Morphogenesis and morphology of HIV. Structure-function relations. Arch Virol. 1989;106(1-2):1–13. doi: 10.1007/BF01311033. [DOI] [PubMed] [Google Scholar]
- Gonda M. A. Bovine immunodeficiency virus. AIDS. 1992 Aug;6(8):759–776. doi: 10.1097/00002030-199208000-00001. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Carter S. G., Kost T. A., Bess J. W., Jr, Arthur L. O., Van der Maaten M. J. Characterization and molecular cloning of a bovine lentivirus related to human immunodeficiency virus. 1987 Nov 26-Dec 2Nature. 330(6146):388–391. doi: 10.1038/330388a0. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Luther D. G., Fong S. E., Tobin G. J. Bovine immunodeficiency virus: molecular biology and virus-host interactions. Virus Res. 1994 May;32(2):155–181. doi: 10.1016/0168-1702(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Gonda M. A. Molecular biology and virus-host interactions of lentiviruses. Ann N Y Acad Sci. 1994 Jun 6;724:22–42. doi: 10.1111/j.1749-6632.1994.tb38893.x. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Benveniste R. E., Sowder R., Copeland T. D., Schultz A. M., Oroszlan S. Molecular characterization of gag proteins from simian immunodeficiency virus (SIVMne). J Virol. 1988 Aug;62(8):2587–2595. doi: 10.1128/jvi.62.8.2587-2595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Bowers M. A., Sowder R. C., 2nd, Serabyn S. A., Johnson D. G., Bess J. W., Jr, Arthur L. O., Bryant D. K., Fenselau C. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processings, and complete amino acid sequences. J Virol. 1992 Apr;66(4):1856–1865. doi: 10.1128/jvi.66.4.1856-1865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R. C., Smythers G. W., Oroszlan S. Chemical and immunological characterizations of equine infectious anemia virus gag-encoded proteins. J Virol. 1987 Apr;61(4):1116–1124. doi: 10.1128/jvi.61.4.1116-1124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Copeland T. D., Smythers G., Oroszlan S. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J Virol. 1984 Nov;52(2):492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hizi A., Henderson L. E., Copeland T. D., Sowder R. C., Krutzsch H. C., Oroszlan S. Analysis of gag proteins from mouse mammary tumor virus. J Virol. 1989 Jun;63(6):2543–2549. doi: 10.1128/jvi.63.6.2543-2549.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. M., Smith H. E., Gregory B., Valli V. E., Whetstone C. A. Detection of multiple retroviral infections in cattle and cross-reactivity of bovine immunodeficiency-like virus and human immunodeficiency virus type 1 proteins using bovine and human sera in a western blot assay. Can J Vet Res. 1992 Oct;56(4):353–359. [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Sherman L., Dahlberg J., Gazit A., Yaniv A., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis of equine infectious anemia virus proviral DNA. Virology. 1987 Jun;158(2):300–312. doi: 10.1016/0042-6822(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Lever A., Gottlinger H., Haseltine W., Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol. 1989 Sep;63(9):4085–4087. doi: 10.1128/jvi.63.9.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Q., Sheridan D., Wood C. Identification and characterization of the bovine immunodeficiency-like virus tat gene. J Virol. 1992 Aug;66(8):5137–5140. doi: 10.1128/jvi.66.8.5137-5140.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Gonda M. A. Conservation of amino-acid sequence motifs in lentivirus Vif proteins. Virus Genes. 1992 Jan;6(1):95–102. doi: 10.1007/BF01703760. [DOI] [PubMed] [Google Scholar]
- Oberste M. S., Greenwood J. D., Gonda M. A. Analysis of the transcription pattern and mapping of the putative rev and env splice junctions of bovine immunodeficiency-like virus. J Virol. 1991 Jul;65(7):3932–3937. doi: 10.1128/jvi.65.7.3932-3937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Williamson J. C., Greenwood J. D., Nagashima K., Copeland T. D., Gonda M. A. Characterization of bovine immunodeficiency virus rev cDNAs and identification and subcellular localization of the Rev protein. J Virol. 1993 Nov;67(11):6395–6405. doi: 10.1128/jvi.67.11.6395-6405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L., Battles J. K., Ennis W. H., Nagashima K., Gonda M. A. Characterization of virus-like particles produced by a recombinant baculovirus containing the gag gene of the bovine immunodeficiency-like virus. Virology. 1990 Oct;178(2):435–451. doi: 10.1016/0042-6822(90)90341-n. [DOI] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Structural role of the matrix protein of type D retroviruses in gag polyprotein stability and capsid assembly. J Virol. 1990 Sep;64(9):4383–4389. doi: 10.1128/jvi.64.9.4383-4389.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rein A. Unmyristylated Moloney murine leukemia virus Pr65gag is excluded from virus assembly and maturation events. J Virol. 1989 May;63(5):2370–2373. doi: 10.1128/jvi.63.5.2370-2373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplin I., Sottong P. Large-volume purification of tumor viruses by use of zonal centrifuges. Appl Microbiol. 1972 May;23(5):1010–1014. doi: 10.1128/am.23.5.1010-1014.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Maaten M. J., Boothe A. D., Seger C. L. Isolation of a virus from cattle with persistent lymphocytosis. J Natl Cancer Inst. 1972 Dec;49(6):1649–1657. doi: 10.1093/jnci/49.6.1649. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]