Abstract
In the present study, a murine ex-vivo somatosensory system preparation was used to determine the response characteristics of cutaneous sensory neurons staining positively for TRPV1 or TRPV2. TRPV1 immunostaining was found exclusively (11/11) in a specific set of mechanically insensitive unmyelinated (C) nociceptors that responded to heating of their receptive fields. No cutaneous C-fibers that responded to both mechanical and heat stimuli stained positively for TRPV1 (0/62). The relationship between TRPV2 and heat transduction characteristics was not as clear, as few unmyelinated or myelinated fibers that responded to heat contained TRPV2. TRPV2 was found most frequently in mechanically sensitive myelinated fibers, including both low threshold and high threshold mechanoreceptors (nociceptors). While TRPV2 was found in only 1 of 6 myelinated polymodal nociceptors, it was found in a majority (10/16) of myelinated mechanical nociceptors. Thus, while the in vivo role of TRPV1 as a heat sensitive channel in cutaneous sensory neurons is clearly defined, the role of TRPV2 in cutaneous neurons remains unknown. These results also suggest that TRPV1 may be essential for heat transduction in a specific subset of mechanically insensitive cutaneous nociceptors, and that this subset may constitute a discrete heat input pathway for inflammation-induced thermal pain.
Perspective
The distinct subset of murine cutaneous nociceptors containing TRPV1 has many attributes in common with mechanically insensitive C-fibers in humans that are believed to play a role in pathological pain states. Therefore these murine fibers provide a clinically relevant animal model for further study of this group of cutaneous nociceptors.
Keywords: mouse, skin, sensory neuron, TRP channel, dorsal root ganglion
Introduction
Cutaneous sensory neurons are a physiologically and neurochemically diverse group. The peripheral response characteristics of these neurons have been the subject of numerous studies across several species. Several studies have shown that correlations exist between response characteristics and other neuronal properties, including conduction velocities, spike shapes, membrane biophysical properties and neurochemical content both in vivo and in vitro.17, 21, 25, 28 Cutaneous sensory neurons are broadly classified into two groups: nociceptors, which are preferentially sensitive to a noxious stimulus or to a stimulus which would become noxious if prolonged, and those that respond to innocuous stimuli such as low threshold mechanoreceptors that encode tactile information.
While the functional properties of these neurons in the transduction of peripheral stimuli of different modalities have been well-studied, the actual receptors/channels responsible for this process have been largely unknown. Recently several landmark studies have identified families of receptors/channels that have the ability to respond to different stimulus modalities.7, 13, 26 One such family is the collection of transient receptor potential (TRP) channels. Two of these channels, TRPV1 and TRPV2, are found in relatively large subsets of primary sensory neurons. Studies employing heterologous expression systems have shown that these channels and other members of the TRP family of channels are capable of responding to a wide range of thermal stimuli as well as protons and pungent chemical compounds such as capsaicin and mustard oil.7, 15 In addition, a recent in vitro study examining dissociated DRG neurons has also shown that many cells that responded to heat also contained TRPV1.12 Likewise, TRPV2 was found to be associated with heat sensitivity, albeit at a much greater temperature than cells containing TRPV1 (43 and 52° C respectively).6 Finally, studies in transgenic mice lacking the TRPV1 receptor (TRPV1-/-) further suggest a functional role for heat detection and the development of heat hyperalgesia following inflammation.5, 10
Previous work from this lab has shown that the most common heat responsive cells innervating the skin, C-polymodal nociceptors (CPM), have normal heat responses in TRPV1-/- mice. Additionally, the vast majority of these afferents with normal heat responses did not stain positively for TRPV2, suggesting that it was not providing the remaining heat sensitivity.35 Therefore, the possible role(s) for these channels in in vivo heat transduction are not clear.
Here we have used an ex vivo skin/nerve/DRG/spinal cord preparation to intracellularly record from and characterize the peripheral response properties of murine cutaneous sensory neurons. Following characterization the cells were injected with dye and histologically recovered and immunostained for either TRPV1 or TRPV2 and/or other markers found in putative nociceptive sensory neurons (e.g. IB4 and CGRP). We found that while TRPV1 immunostaining is restricted to a subset of cutaneous nociceptors, TRPV2 immunostaining was found in many different fiber types, including non-nociceptors.
Materials and Methods
All procedures used in these experiments were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and followed the guidelines of the International Association for the Study of Pain.
Ex-vivo preparation
The ex-vivo somatosensory system preparation has been described in detail previously.34 Briefly, adult mice (Swiss Webster, C57/Bl6 and TRPV1(-/-)) were anesthetized via intramuscular injection of ketamine and xylazine (90 and 10 mg/kg, respectively) and perfused transcardially with oxygenated (95% O2-5% CO2) artificial CSF(aCSF; in mM: 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 D-glucose) with 253.9 mM sucrose at 12-15°C. Two preparations were used in these studies: one consisted of thoracic spinal cord, thoracic DRGs with associated dorsal cutaneous nerves (DCNs) and back skin, and the other was lumbar spinal cord and DRGs (L3, L2), saphenous nerve and foot dorsum skin. Following dissection, the preparation was transferred to a separate recording chamber containing chilled oxygenated aCSF in which the sucrose was replaced with 127.0 mM NaCl. The skin was pinned out on a stainless steel grid located at the bath/air interface, such that the dermal surface remained perfused with the aCSF while the epidermis was exposed to the air. The platform provided stability during applied thermal and mechanical stimuli. The bath was then slowly warmed to 31°C before recording.
Recording and Stimulation
Sensory neuron somata were impaled with quartz glass microelectrodes (impedance >100MΩ) containing 5-10% Neurobiotin (Vector Laboratories, Burlingame, CA) in 1 M potassium acetate. Orthograde electrical search stimuli were delivered through a suction electrode on the saphenous nerve to locate sensory neuron somata with a peripheral axon innervating the prepared skin. Peripheral receptive fields (RF) were localized with a blunt glass stylus and von Frey hairs. When cells were driven by the nerve but had no mechanical RF, a thermal search was conducted. This was accomplished by applying hot (∼52°C) and/or cold (∼ 0°C) physiological saline to the skin using a 10 ml syringe with a 20 gauge needle. If a thermal RF was located, the lack of mechanical sensitivity was confirmed by searching the identified RF using a glass rod.
The response characteristics of the DRG cell were determined by applying computer controlled mechanical and thermal stimuli. The mechanical stimulator consisted of a tension/length controller (Aurora Scientific) attached to a 1mm diameter plastic disc. Computer controlled 5s square waves of 1, 5, 10, 25, 50 and 100 mN were applied to the cell’s RF. Mechanical threshold was determined to be the lowest stimulus intensity of this series to elicit at least one action potential.
After mechanical stimulation, a controlled thermal stimulus was applied using a 3mm2 contact area peltier element (Yale Univ. Machine Shop). The temperature stimulus consisted of a 15s heat ramp from 31-52° C followed by a 5s plateau at 52°. The stimulus then ramped back down to 31°C in 15s. In many cases while recording from myelinated nociceptors multiple heat applications were made and in some cases the heat ramp was continued to 54°C and held for 5s. The thermal threshold was determined to be the temperature during the heat ramp at which the first action potential was elicited. All elicited responses were recorded digitally for offline analysis (Spike2 software, Cambridge Electronic Design).
After physiological characterization, the cell was labeled by injecting Neurobiotin iontophoretically (2-3 cells per DRG). Peripheral conduction velocity was calculated from spike latency and the distance between the stimulating and recording electrodes (measured directly along the nerve).
Tissue processing and analysis of recorded cells
Once a sensory neuron was characterized and filled with Neurobiotin, the DRG containing the injected cell was removed and immersion fixed (4% paraformaldehyde in 0.1 M PB for 30 min at 4°C). Ganglia were then blocked, embedded in 10% gelatin, postfixed overnight, and cryoprotected in 20% sucrose. Frozen sections (60 μm) were collected in PB, and reacted with primary antisera for either TRPV1 (Calbiochem; San Diego, CA), TRPV2 (primary antibody provided by M.J.Caterina.) or CGRP (rabbit anti-CGRP; Chemicon; Temecula, CA or guinea pig anti-CGRP; Research Diagnostic Inc.; Flanders, N.J.). In most cases this was combined with IB4 staining (Molecular Probes; Eugene, OR). After incubation in primary antiserum, tissue was washed and incubated in donkey anti-rabbit secondary antiserum (conjugated to Cy2, Cy3, or Cy5, Jackson Immunoresearch; West Grove, PA), and reacted with fluorescently-tagged avidin to label Neurobiotin-filled cells (Vector Laboratories: Burlingame, CA). Distribution of fluorescent staining was determined using an Olympus confocal microscope and software (Fluoview). Sequential scanning was done to prevent bleed-through of the different fluorophores. The TRPV1 antibody used in these studies was tested using lumbar DRGs from TRPV1-/- mice and there was no visible staining (data not shown). In addition, the TRPV2 antibody did not stain DRG cells taken from TRPV2-/- mice (personal communication from Dr. Michael Caterina).
Data analysis
Student’s t-tests were used to analyze different aspects of the responses of neurons to both mechanical and heat stimuli. This information was sorted by neuronal functional type to examine whether or not certain classes of neurons have coherence with regard to the expression of any of the markers tested. In the analysis of mean firing rate/°C in response to the application of the addition a two-way ANOVA analysis was completed and followed with Boniferroni post hoc analysis.
RESULTS
Classification and distribution of cutaneous sensory neurons
A total of 402 primary cutaneous neurons were intracellularly recorded and physiologically characterized from 97 WT mice. The bulk of the neurons examined were from hindlimb-saphenous nerve preparations (335) with the rest coming from back skin-dorsal cutaneous nerve preparations (67). Neurons were sorted into classes depending upon their conduction velocities and response characteristics. Neurons with a conduction velocity of < 1.2 m/s were classified as C-fibers, and all others were classified as A fibers.18, 19 Myelinated fibers were divided into two groups, low threshold mechanoreceptors and high threshold mechanoreceptors (nociceptors) based on mechanical response properties and somal spike shapes. These fibers also exhibited response properties very similar to those described in previous studies. In addition to clear differences in their peripheral response properties, myelinated nociceptors also had characteristic broad action potentials with inflections on the falling phase of the spike.17 The durations of their APs (measured at half amplitude) were significantly different from those of myelinated low threshold mechanoreceptors (1.55 ± 0.15 ms vs. 0.58 ± 0.13; p<0.001) recorded in the same preparations. This result is consistent with previous studies in cat and rat.17, 28
The low threshold mechanoreceptors were divided into three groups: 1) A-β rapidly adapting (guard-hair afferents), 2) slowly adapting Type 1, 3) A-δ rapidly adapting down-hair afferents. A-fiber nociceptors were sorted into three categories: APM (A-polymodal, responds to mechanical and heat stimuli), AM (A-mechanical only), and AMC, (responds to both mechanical stimuli and cooling of the skin), with AM fibers the most frequently encountered type. These fibers had a very large range of conduction velocities (1.4-14.5 m/s) spanning both the A-δ and A-β CV groups. APM and AM neurons had mechanical thresholds that were statistically indistinguishable (16 ± 5.1 vs. 27.8 ± 4.7 mN). The distribution of these fibers types can be seen in figure 1A.
Figure 1.

Distribution of physiologically characterized cutaneous sensory neurons in normal (WT) mice. Rapidly adapting low threshold mechanoreceptors (LTMRs) made up the most significant portion of the myelinated afferents (A), whereas C-Polymodal fibers made up the bulk of the unmyelinated population (B).
The C-fiber classes included: 1) C-polymodal (CPM), which responded to mechanical and heat and sometimes cool/cold stimuli 2) C-mechano (CM), which responded only to mechanical deformation of the cell’s RF; 3) C-mechano cool/cold (CMC), which responded to mechanical and cooling (but not heating); 4) C-heat (CH), which responded only to heat stimuli. 5) C-cooling/cold only (CC), which responded only to lowering skin temperature. The distribution of C-fiber types can be seen in figure 1B. Only fibers recorded in the saphenous nerve preparations were used to construct this distribution as we did not adequately search for mechanically insensitive, but thermally responsive cells in the studies using the back skin preparation. In agreement with previous studies in several different species the largest group of cutaneous C-fibers was that classified as polymodal nociceptors (72%). The CM (11%), CH (9%), CMC (7%) groups of fibers were encountered in similar numbers, and the most infrequently encountered group of was those fibers only responding to cooling (CC) of the skin (1%).33 We encountered a number of cells that were driven by the nerve stimulus, but were found to be both mechanically and thermally unresponsive (not shown). However, only cells that had a response to cutaneous stimulation were included in the distribution seen in figure 1B.
Quantitative physiological characterization of RFs
Overall the different C-fiber groups had statistically similar mechanical and thermal thresholds. For example, the average heat threshold for the CPMs was 41.5 ± 0.4°C and for CH fibers it was 42.2 ± 2.3°C. The average mechanical threshold was 16.9 mN ± 1.7 for CPM fibers, 17.8 mN ± 5.0 for CM fibers, and 20.6 mN ± 12.0 for the CMC group. It should be noted that the CMC group included cells with a broad range of mechanical and thermal thresholds. Some responded to innocuous mechanical (1mN) and cooling (∼ 28°C) while others responded to noxious stimulus intensities for one modality and innocuous intensities for the other. Finally, others only responded to stimuli in the noxious range for both modalities.
As described in the Methods, when a cell was activated by the electrical search stimulus and did not have a mechanical receptive field we would use noxious hot (∼ 52°C) and/or cold (∼ 0°C) saline to search for thermally sensitive fibers. This strategy raises the possibility that during the experiments the brief, but multiple heating and cooling of the skin could alter the response properties of these fibers. To determine if this was the case, we compared the mean thermal and mechanical thresholds for the first 2 fibers characterized in an experiment with the last 2 fibers for all the experiments involving WT (SW and C57Bl6) mice. We found no differences in the heat thresholds (first two cells = 39.3 ± 0.8 vs last 2 = 40.3 ± 1.1°C, p = 0.5), and mechanical thresholds, (16.9 ±2.8 vs 14.1 ± 3.5 mN, p = 0.4) or any of the other measures (e.g. conduction velocity, mean firing rate) suggesting that our search strategy was not significantly affecting the response properties of the afferent fibers.
Distribution of immunolabeled cells
Of the total of 402 afferent fibers characterized, 186 were intracellularly labeled, recovered and immunohistochemically characterized. Of these 63 were from back skin and 123 were from saphenous (hindlimb) preparations. While most sections were stained with antisera to TRPV1 or TRPV2, given the reported size distributions of primary sensory neurons immunopositive for these channels5 most TRPV2 staining was carried out on myelinated A-fibers and TRPV1 staining on unmyelinated C-fibers. In addition, in the majority of cases (87%) identified C-fibers were stained for TRPV1 content and IB4 binding. We recovered 23 stained low threshold mechanoreceptors (G-hair = 7; SA1 = 6; D-hair =10), to determine if they contained TRPV2 and/or CGRP. The remaining 163 were classified as nociceptors with 130 C-fiber nociceptors (CPM = 85; CM = 16; CMC = 18; CH = 11) and 33 A-fiber nociceptors (APM = 13; AM = 19; AMC = 1). Results of the immunostaining are summarized in Table 1.
Table 1. Immunostaining results for wildtype A and C cutaneous neurons. nt: not tested.
| Neuron Type | Number Stained | IB4+ | TRPV1+ | CGRP+ | TRPV2+ |
|---|---|---|---|---|---|
| SA1 | 6 | nt | 0/1 | 0/2 | 1/6 |
| D-hair | 10 | 0/1 | nt | 0/5 | 0/9 |
| G-Hair | 7 | nt | nt | 0/4 | 3/7 |
| AM | 19 | 0/1 | nt | 6/9 | 10/16 |
| APM | 13 | 1/1 | 1/7 | 0/6 | 1/6 |
| AMC | 1 | nt | nt | nt | 1/1 |
| CH | 11 | 0/9 | 11/11 | nt | nt |
| CM | 16 | 5/9 | 0/11 | 1/4 | 1/6 |
| CMC | 18 | 0/13 | 0/4 | 1/8 | 2/6 |
| CPM | 85 | 55/71 | 0/62 | 2/13 | 2/12 |
TRPV2 is not well correlated with heat sensitivity
A total of 69 physiologically characterized neurons were examined for TRPV2 immunostaining. In contrast to the finding for TRPV1, the TRPV2 immunopositive cells were quite heterogenous in their functional properties. TRPV2 positive-stained cells were found in both myelinated (Fig. 2) and unmyelinated fiber groups, and responded to both low (innocuous) (Fig. 2A) and high (noxious) threshold mechanical stimuli (Fig. 2B). There were, however, some differences in the percentage of these different types of fibers that contained TRPV2. TRPV2 staining was most frequently found in myelinated nociceptive sensory neurons (63%) and next in Aβ rapidly adapting low-threshold fibers (43%). It was surprising to find that only a small number (3/21) of TRPV2 immunopositive cells responded to heat. In order to test the possibility that these fibers were only sensitive to temperatures >52°C, or that they would only respond to heat if first sensitized by multiple heatings, we repeated the heat stimulus multiple times raising the plateau temperature to 54°C. We were unable to induce a response to heat in any of those fibers that did not respond to the initial heat stimulus. It was also interesting to find that the few thermally sensitive TRPV2 positive fibers (myelinated and unmyelinated) were just as likely to respond to cold stimuli (3/6) as they were to heat (3/6). Thus the strongest functional correlate with TRPV2 immunostaining was found to be mechanical sensitivity as they all had mechanical receptive fields.
Figure 2.
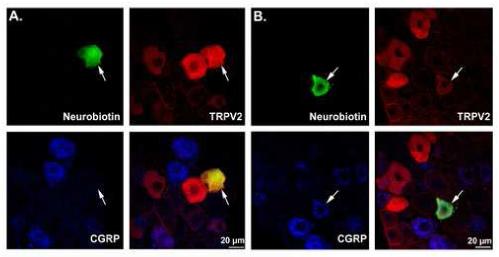
TRPV2 immunoreactivity in two myelinated cutaneous neurons. (A) a low threshold mechanoreceptor (hair follicle afferent) that was positive for TRPV2 but not CGRP. (B) a high threshold mechanoreceptor (A-mechano, AM) that was positive for both CGRP and TRPV2. TRPV2 immunoreactivity was a poor predictor of heat sensitivity.
This correlation and the fact that TRPV2 staining was found in some low-threshold mechanoreceptors suggested that TRPV2 may have some effect on mechanical sensitivity in the population of myelinated nociceptors. Although we found a trend for TRPV2-positive myelinated nociceptors to have lower mechanical thresholds than the TRPV2-negative ones, this difference did not reach statistical significance (mean thresholds TRPV2-positive = 20.0 ± 17.8 mN; TRPV2-negative = 38.3 ± 26.7 mN). We also found that there were no differences in the firing rates between these two groups.
TRPV1 staining identified a distinct subset of heat sensitive cutaneous neurons
A total of 86 characterized cutaneous C-fiber somata were reacted with primary antisera for TRPV1. Of these only 11 were found to be immunoreactive for TRPV1. All 11 of these cells were characterized as mechanically insensitive CH fibers (Fig. 3) and all of the recovered CH neurons stained positively for TRPV1. We also looked for TRPV1 staining in a limited number of myelinated cutaneous afferents primarily those classified as APM fibers. Only 1 of the 6 APM tested was found to be immunopositive for TRPV1. This one A-fiber, when added to the TRPV1-positive C-fibers, suggest that most if not all cutaneous fibers that contain TRPV1 are responsive to heat.
Figure 3.

Example of the comprehensive phenotype of a mechanically insensitive C-Heat (CH) neuron. As for all CH neurons the NB-stained cell (A) stained positively for TRPV1 (B) but, was IB4 negative (C). This fiber was mechanically insensitive (not shown) but, responded to heat (D). CH neurons were the only cutaneous neurons examined that were immunopositive for TRPV1.
The distribution of isolectin B4 binding also turned out to denote a rather homogeneous set of cutaneous C-fibers. A total of 55 of the 71 (77%) CPM (Fig. 4) and 5 of 9 (55%) CM somata recovered and tested bound IB4. All CMC fibers and TRPV1 positive CH fibers were IB4 negative (Fig. 3). The finding that positive TRPV1 staining and IB4 binding were found in functionally different subsets of cutaneous C-fibers is in agreement with previous studies showing a lack of co-localization of these markers in mice.36
Figure 4.

Example of the comprehensive phenotype of a C-Polymodal (CPM) neuron. This neuron NB-stained cell (A) was negative for TRPV1 (B) but, positive for IB4 (C) and responded to mechanical and heat stimuli (D).
As stated above, TRPV1 immunoreactivity was found only in CH cutaneous neurons. Comparison of the physiological properties of TRPV1 positive and negative neurons revealed that they have differences other than submodality classification. CH neurons were distinguished from other C-fibers by their significantly lower mean conduction velocity (CH = 0.351 ± 0.02 m/s all other C-fibers = 0.561 ±.01 m/s; p < 0.001) (Fig. 5A). Like the CPM fibers, the CH fiber responses comprise a heterogeneous group with regards to their individual heat thresholds, but the mean thresholds between the two groups were not different (Fig. 5B). However, the intensity of their responses to the heat stimulus was significantly different. This difference was reflected in significantly higher mean firing rates at noxious temperatures (Two-way ANOVA, Bonferroni p < 0.05; Fig. 5C) and the mean peak firing rate (at the temperature eliciting the greatest response) when compared to the CPM fibers (Fig. 5D).
Figure 5.

Physiological characteristics of different submodalities of cutaneous neurons. (A) C-Heat only (CH) neurons had significantly lower conduction velocities than any other group. B. Thermal thresholds of C-polymodal (CPM) neurons were indistinguishable from CH neurons. (C) Mean firing rates of all CH and CPM neurons during the heat ramp stimulus. Even though mean thresholds in (B) are identical, the dynamic response profiles during the stimulus are markedly different. (D) Neurons in each group were maximally excited at different temperatures during the heat stimulus, and the firing rates at these temperatures were averaged. The mean maximal firing rate of CH neurons is significantly higher than that of CPM neurons (* p<.01, ** p<.001).
TRPV1-/- mice lack CH fibers
In a previous study we determined that in TRPV1-/- mice CPM fibers exhibited normal mechanical and thermal response properties. Given these findings presented above we reexamined TPRV1-/- mice to determine whether the heat response of CH fibers was adversely affected by the loss of TRPV1. We characterized 170 cutaneous afferents in 29 TRPV1 -/- mice. Of these, 59 were myelinated A-fibers and 111 were C-fibers, of which 81 were classified as CPM, 11 CM, 16 CMC and 3 CC. Consistent with our earlier study35 we found no differences in the heat response recorded from CPM nociceptors in WT and TRPV1-/- mice (e.g. WT heat threshold = 41.5 ± 0.41°C; TRPV1-/- 41.2 ± 0.68°C). In addition, there were also no differences observed in the mechanical sensitivity of C-fibers in the TRPV1 mice (e.g. WT = 16.9 ± 1.7 mN; TRPV1-/- = 13.9 ± 2.4 mN). Surprisingly, we did not find any CH fibers in these mice. In addition, this loss was coupled with an apparent increase in fibers that were specifically sensitive to cold stimuli (Fig. 6). However, this trend was not significantly different from WT values (p = 0.07, Chi-square). These results suggest that TRPV1 is essential for heat sensitivity in the CH fibers.
Figure 6.

Distribution of physiologically characterized cutaneous sensory neurons in normal TRPV1-/- mice. Rapidly adapting low threshold mechanoreceptors (LTMRs) and A-mechano nociceptors (AM) made up the most significant portion of the myelinated afferents (A), whereas C-Polymodal fibers made up the bulk of the unmyelinated population (B). Note that there are no CH fibers in the unmyelinated population (compare with Figure 1).
Discussion
The discovery of the family of transient receptor potential channels has greatly energized research into the molecular basis of sensory transduction. In studies employing heterologous expression systems, several of these channels have been shown to be sensitive to specific thermal stimuli.16 However, little is known about the functional properties of these channels in vivo. Here we have correlated the response properties of characterized cutaneous sensory neurons with immunoreactivity for two of these channels. We found that TRPV1 immunoreactivity was highly correlated with a relatively small subset of cutaneous sensory neurons that were responsive to heat, but insensitive to mechanical stimulation. On the other hand, TRPV2 staining was found in a wide variety of cutaneous afferents and there was very little correlation with heat sensitivity.
All of the fiber types observed in these studies have been found previously in one or more species.33 The distribution of fiber types found here is quite similar to that seen in other studies examining fibers in the saphenous nerve in both rat19, 22 and mouse.3, 18 While fibers responding to mechanical stimulation have been studied in great detail in these and other studies, mechanically insensitive afferents that respond only to thermal stimuli have not been routinely searched for or examined in rodents. One study that did routinely search for thermally sensitive fibers found slightly different proportions than seen here with CC fibers outnumbering CH fibers.19 These differences could be the result of species differences or differences in the techniques used isolate individual sensory neurons. For example the CC fibers have significantly faster CVs than the CH fibers suggesting a larger fiber diameter which could result in an increased probability of isolation using the fiber teasing approach. Regardless of any differences in the relative distribution of fiber types, we are confident that we have been able to determine the extent of immunostaining for TRPV1 and TRPV2 in all of the different cutaneous fiber types innervating mouse hairy skin.
In the initial report on TRPV2 it was suggested that it may be located in myelinated nociceptors possibly providing heat sensitivity to those fibers.6 It was also suggested that these would likely be type1 myelinated mechanoheat fibers based on the high heat thresholds of these fibers in primates.31 The results presented here have confirmed that a majority (62.5%) of myelinated mechanical nociceptive fibers (AM) did stain positively for TRPV2. However, those responding to both to mechanical and heat stimuli (APM) rarely (17%) stained positively for TRPV2. Indeed, the results presented here suggest that mechanical sensitivity is the strongest functional correlate of TRPV2 immunostaining. The possibility of TRPV2 playing a role in mechanotransduction is consistent with TRPV2’s homology with the osmolarity and mechanically sensitive calcium channel osm-9 found in C. elegans.9, 14 However, the heterogenic distribution of fiber types (e.g low and high threshold mechanoreceptive with and without thermal sensitivity) immunopositive for TRPV2 also suggests the possibility of a more ubiquitous function in cutaneous sensory neurons.
Earlier studies in rabbits, cats11 and primates4 have shown that following repeated heating of their mechanical receptive fields, myelinated nociceptors could be sensitized to respond to heat. Although a subsequent study showed that myelinated nociceptors in rat were rarely sensitized to respond to heat,23 it was still plausible that murine AM-fibers could be sensitized to respond to heat and that TRPV2 might play a role in this process. In this regard, a recent report in rat 27 has shown that following dissociation, cells containing TRPV2 show sensitization to repeated heating. Therefore, we were careful to examine this possibility. We were unable to induce any of these fibers to respond to heat, which is consistent with the results from rat. However, we cannot rule out the possible contribution of TRPV2 to heat sensitivity following peripheral injury, or cell dissociation.
While it is not possible to ascertain the in vivo functional (sensory transduction) properties of the TRPV2 channel in these studies, it is clear that somal TRPV2-positive immunoreactivity is not a good predictor of heat sensitivity in the cutaneous terminals.
Of all the different members of the TRP family TRPV1 has clearly been the one most extensively studied. TRPV1 has been shown to be responsive to capsaicin, heat, and protons providing an interesting example of the potential for modality convergence in a single cutaneous sensory neuron. Therefore while we expected to find that the cells unresponsive to heat, i.e., CM, CMC, did not stain positively for TRPV1, it was quite surprising to find that none of the 62 heat-sensitive CPM fibers examined stained positively for TRPV1. These results strongly suggest that the TRPV1 channel is found only in a small group of modality specific (heat) cutaneous C-fibers. It was also of interest that such large proportion of the CPM fibers bound IB4 (77%) and that relatively few stained positively for CGRP (13%). These findings are consistent with previous reports suggesting that a higher proportion of C-fibers innervating glabrous skin staining positively for CGRP than in hairy skin.20, 21 Taken together with previous findings showing a high degree of co-localization of TRPV1and CGRP35, 36, our findings would suggest that roughly half of the peptidergic fibers C-fibers innervating mouse hairy skin are mechanically insensitive CH fibers.
The finding that TRPV1 is in a small percentage of cutaneous C-fibers is apparently at odds with a previous study examining capsaicin sensitivity in dissociated DRG cells.2 That is, the results presented here would suggest that in cell culture studies only a small percentage of cutaneous sensory neurons would respond to capsaicin and that very few if any of the cells that bound IB4 would be responsive. This is not the case as a small but significant percentage of capsaicin responders bind IB4 in vitro. However, it should be pointed out that in cell culture studies the identity of the peripheral target of sensory neurons in lost. It is quite possible that many of the cells examined in culture had innervated non-cutaneous tissues. For example, a much higher percentage of C-fibers innervating muscle stain positive for TRPV1 C-fibers innervating skin.8 In addition, it is not clear that IB4 is necessarily a permanent marker for specific cell types following injury or cell dissociation.
One of the more interesting possibilities suggested from the results presented here is the similarity between the TRPV1 positive CH fibers found in the mouse and nonhuman primates1 and the mechanically insensitive C-fibers (MIA) found in humans.29 Studies employing microneurography recording techniques in humans have shown that this group of mechanically insensitive C-fibers has distinct biophysical properties32. As a group they have significantly slower conduction velocities and exhibit profound conduction velocity slowing with repeated activity. It should be pointed out that while only some of these MIA fibers were found to be sensitive to heat, it was not possible to test higher temperatures in the human subjects. In addition, while many afferent fibers were found to be responsive to cutaneous injections of capsaicin, only the MIA fibers had responses that were well correlated in magnitude and duration with the reported pain perception.30 Similar results were also reported in nonhuman primate where only CH fibers responded to capsaicin injections in a manner that closely matched the magnitude and duration of pain in humans.1, 30 Finally, in microneurography recordings from patients suffering from a chronic neuropathic pain condition (Erythromelalgia)24, it was reported that the fibers with biophysical properties similar to those of mechanically insensitive fibers were those most prone to developing spontaneous activity and in some cases developed mechanical sensitivity. They concluded that this population of fibers was actively contributing to the chronic pain state. Thus the TRPV1-positive CH fibers found in mouse may be a valuable model for the study of the contribution of these capsaicin sensitive fibers to persistent pain states.
In the initial study of the TRPV1-/- mice5 it was shown that while mechanical allodynia developed following inflammation in these transgenic mice, they did not develop heat hyperalgesia. However, in a model of neuropathic pain the mice developed both heat and mechanical hyperalgesia. Therefore, findings presented here showing a lack of CH fibers in TRPV1-/- mice suggest that these normally mechanically insensitive sensory neurons play an essential role in the development of inflammation induced heat hyperalgesia. Whereas it is clear that nociceptors containing TRPV1 are necessary for the development of heat hyperalgesia after CFA injections, it is unclear whether this is due to a lack of CH fibers or that both CH and CPM fibers are necessary. The results presented here further suggest that mechanical hyperalgesia observed following inflammation is either due to sensitization of mechanically sensitive fibers (e.g. CPM, CM), the acquisition of mechanical sensitivity by previously insensitive fibers, or that there is a significant central component to the altered sensitivity. It will be important to examine these issues directly using the TRPV1-/- mice.
In conclusion, TRPV1 and TRPV2 have been shown to be sensitive to heat in in vitro heterologous expression systems and dissociated cell culture studies; however, the ex vivo studies performed here suggest that TRPV1 may be important for heat transduction in a very limited subset of cutaneous neurons. In addition this population of fibers shares similarities with the population of mechanically insensitive C-fibers in humans believed to play a significant role in chronic pain states. While TRPV2 is expressed in a large assortment of mechanically sensitive cutaneous sensory neurons, it is not well correlated with heat sensitivity in any myelinated or unmyelinated group as defined here.
Acknowledgements
We thank Weiwen Wang, Collene Anderson and Julie Kopsak for excellent technical assistance. Supported by NIH grants NS23725 (H.R.K) and NS44094 (C.J.W.) C.J.W.’s present address is Department of Zoology and Physiology, University of Wyoming, Laramie, WY 82071.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 2.Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol. 2001;85:1561–1574. doi: 10.1152/jn.2001.85.4.1561. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JN, Meyer RA, LaMotte RH. Sensitization of myelinated nociceptive afferents that innervate monkey hand. J Neurophysiol. 1979;42:1669–1679. doi: 10.1152/jn.1979.42.6.1669. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 8.Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald M, Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. J Physiol. 1977;265:549–563. doi: 10.1113/jphysiol.1977.sp011730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galoyan SM, Petruska JC, Mendell LM. Mechanisms of sensitization of the response of single dorsal root ganglion cells from adult rat to noxious heat. Eur J Neurosci. 2003;18:535–541. doi: 10.1046/j.1460-9568.2003.02775.x. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Anoveros J, Corey DP. The molecules of mechanosensation. Annu Rev Neurosci. 1997;20:567–594. doi: 10.1146/annurev.neuro.20.1.567. [DOI] [PubMed] [Google Scholar]
- 14.Goodman MB, Schwarz EM. Transducing touch in Caenorhabditis elegans. Annu Rev Physiol. 2003;65:429–452. doi: 10.1146/annurev.physiol.65.092101.142659. [DOI] [PubMed] [Google Scholar]
- 15.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 16.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 17.Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- 18.Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 19.Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- 20.Lawson SN. Peptides and cutaneous polymodal nociceptor neurones. Prog Brain Res. 1996;113:369–385. doi: 10.1016/s0079-6123(08)61099-7. [DOI] [PubMed] [Google Scholar]
- 21.Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- 22.Lynn B, Carpenter SE. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982;238:29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- 23.Lynn B, Shakhanbeh J. Properties of A delta high threshold mechanoreceptors in the rat hairy and glabrous skin and their response to heat. Neurosci Lett. 1988;85:71–76. doi: 10.1016/0304-3940(88)90431-4. [DOI] [PubMed] [Google Scholar]
- 24.Orstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jorum E, Handwerker H, Torebjork E. Pathological C-fibres in patients with a chronic painful condition. Brain. 2003;126:567–578. doi: 10.1093/brain/awg060. [DOI] [PubMed] [Google Scholar]
- 25.Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol. 2000;84:2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- 26.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 27.Rau KK, Jiang N, Johnson RD, Cooper BY. Heat sensitization in skin and muscle nociceptors expressing distinct combinations of TRPV1 and TRPV2 protein. J Neurophysiol. 2007;97:2651–2662. doi: 10.1152/jn.00840.2006. [DOI] [PubMed] [Google Scholar]
- 28.Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 31.Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol. 1998;80:1082–1093. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- 32.Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. Kluwer Academic/Plenum Publishers; New York: 2004. [Google Scholar]
- 34.Woodbury CJ, Ritter AM, Koerber HR. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J Comp Neurol. 2001;436:304–323. [PubMed] [Google Scholar]
- 35.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


