Abstract
The predominance of rivalrous targets is affected by surrounding context when stimuli rival in orientation, motion or color. This study investigated the influence of chromatic context on binocular color rivalry. The predominance of rivalrous chromatic targets was measured in various surrounding contexts. The first experiment showed that a chromatic surround's influence was stronger when the surround was uniform or a grating with luminance contrast (chromatic/black grating) compared to an equiluminant grating (chromatic/white). The second experiment revealed virtually no effect of the orientation of the surrounding chromatic context, using chromatically rivalrous vertical gratings. These results are consistent with a chromatic representation of the context by a non-oriented, chromatically selective and spatially antagonistic receptive field. Neither a double-opponent receptive field nor a receptive field without spatial antagonism accounts for the influence of context on binocular color rivalry.
Keywords: binocular color rivalry, chromatic-surround influence, separate processing of color and form, receptive-field organization
Introduction
Binocular rivalry is alternation between two percepts of different stimuli presented to the two eyes (Blake & Logothetis, 2002). Binocular color rivalry occurs when two sufficiently different chromaticities are presented dichoptically to the same part of the visual field. Binocular color mixture is generally observed when the difference is small (Ikeda & Sagawa, 1979). Perceptual alternation between two rivalrous stimuli occurs spontaneously and involuntarily, but the temporal dynamics of the alternation can be modulated by several factors such as contrast (Levelt, 1965), motion (Blake, Sobel & Gilroy, 2003), attention (Chong & Blake, 2006) and perceptual grouping (Kovács, Papathomas, Yang & Fehér, 1996; Alais & Blake, 1999).
Rivalry between two gratings with different orientations depends also on stimulation in the area surrounding the rivalrous targets (Fukuda & Blake, 1992; Carter, Campbell, Liu & Wallis, 2004; Paffen, Tadin, te Pas, Blake & Verstraten, 2006). For example, when rivalrous targets with perpendicular orientations are both surrounded by a grating with one of the orientations, the duration of predominance decreases for the target with the same orientation as the surround. This suppressive influence of the surround has been reported also for motion rivalry (Paffen, te Pas, Kanai, van der Smagt & Verstraten, 2004; Paffen, Alais & Verstraten, 2005; Paffen et al., 2006) and color rivalry (Carter et al., 2004; Paffen et al., 2006).
Inhibitory center-surround interaction among motion-selective neurons is proposed as the underlying mechanism for the suppressive-surround influence during motion rivalry (Paffen et al. 2004, 2005, 2006). For color, single cell recording reveals that a chromatically selective cortical cell can be suppressed by a chromatic surround (Solomon, Peirce & Lennie, 2004). In general, several different types of chromatically selective cells are found in early visual cortex. They can be either orientation selective or non-orientation selective, and also respond selectively either to only chromatic modulation or to both chromatic and/or luminance modulation (Johnson, Hawken & Shapley, 2001; Conway, 2001). The experiments here investigated which of these cell types might mediate the chromatic-surround influence on binocular color rivalry.
Psychophysical studies are consistent with cells having both color and orientation selectivity (Clifford, Pearson, Forte & Spehar, 2003; Clifford, Spehar, Solomon, Martin & Zaidi, 2003). The perceived orientation of a central grating is affected by the orientation of the surrounding grating, with the greatest influence of the surround when center and surround are modulated along the same axis in color space. While these psychophysical measurements imply that a surround's influence on perceived orientation depends on the chromaticity of the surround, they do not provide evidence that a surround's influence on color perception depends on the surround's spatial features.
The first experiment of the current study varied chromatic contrast and luminance contrast within the surround, using uniform but chromatically rivalrous central fields. The design assessed the type(s) of receptive-field organization consistent with chromatic-surround influence, regardless of orientation selectivity. The second experiment investigated whether the influence of a chromatic surround on binocular color rivalry depended on spatially congruent forms in the surround and rivalrous targets: the orientation of the surround and targets was either identical or orthogonal. If the neural representation mediating the chromatic-surround influence is not selective for orientation, the influence of the chromatic surround on binocular color rivalry should be the same for both orientations of the surround. On the other hand, if an orientation-selective neural representation of the surround mediates the surround's influence, the reduced duration of predominance caused by presenting the same chromaticity in center and surround should be greater when the orientation of the surrounding and rivalrous gratings is the same compared to when they are perpendicular.
Method
Apparatus
All stimuli were generated using a Macintosh G4 computer, and presented on an accurately calibrated Sony color display (GDM-F520). The R, G and B guns of the color cathode-ray tube (CRT) were driven by a Radius ThunderPower 30/1600 video card with 10-bit resolution; the guns' spectral power distributions were measured using a spectroradiometer (Photo Research PR-650). The relative light level of each gun at every digital value (1024; 210 levels) was measured with an International Light radiometer/photometer (model IL-1700). These values were saved in a lookup table. Absolute luminance and the stability of the calibration were measured frequently with a Minolta LS-100 photometer. The color CRT had 1360 × 1024 pixel resolution at a refresh rate of 75 Hz non-interlaced.
Two stimuli presented on the CRT screen were projected separately to the two eyes through a haploscope. The haploscope was composed of eight front-surface mirrors. Two of the mirrors were attached to a saddle on a triangular rail, which allowed observers to adjust its position for their individual interocular distance. A chin rest was used to stabilize head position.
Stimuli
The influence of chromatic context on color rivalry was measured by systematically varying the chromaticities of the center and surround presented to each eye. Four pairs of chromaticities, specified within a cone-excitation space (MacLeod & Boynton, 1979), provided consistent color rivalry (Fig. 1a). Two of the pairs had both L- and S-cone contrast (Pair 1: [L/(L+M)=0.718, S/(L+M)=3.3] and [L/(L+M)=0.611, S/(L+M)=0.3]; Pair 2: [L/(L+M)=0.718, S/(L+M)=0.3] and [L/(L+M)=0.611, S/(L+M)=3.3]). One had no S-cone difference (Pair 3: [L/(L+M)=0.718, S/(L+M)=1.0] and [L/(L+M)=0.611, S/(L+M)=1.0]), and one had only an S-cone difference (Pair 4: [L/(L+M)=0.667, S/(L+M)=3.3] and [L/(L+M)=0.667, S/(L+M)=0.3]). The arbitrary unit of S/(L+M) was normalized here to 1.0 for equal-energy-spectrum ‘white’ (EES; circle in Fig. 1a) 1.
Figure 1.
(a) Chromaticities used in the experiments, plotted in a modified Macleod-Boynton diagram (MacLeod & Boynton, 1979). Four pairs of chromaticities (connected by arrows) were used (chromaticity-pair number shown within each square). Pair 1: (L/(L+M)=0.718, S/(L+M)=3.3) and (L/(L+M)=0.611, S/(L+M)=0.3), Pair 2: (L/(L+M)=0.718, S/(L+M)=0.3) and (L/(L+M)=0.611, S/(L+M)=3.3), Pair 3: (L/(L+M)=0.718, S/(L+M)=1.0) and (L/(L+M)=0.611, S/(L+M)=1.0), Pair 4: (L/(L+M)=0.667, S/(L+M)=3.3) and (L/(L+M)=0.667, S/(L+M)=0.3). EES is shown by a circle (L/(L+M)=0.667, S/(L+M)=1.0). (b) An example of a stimulus configuration in Experiment 1. (c) An example of a stimulus configuration in Experiment 2. In both experiments, the size of the central rivalrous target was 1 deg and the gap between the target and the surround was 0.1 deg.
The rivalrous stimulus was in a 1 deg diameter circular aperture surrounded by an annulus with inner/outer diameter 1.2/2.0 deg (Figs. 1b, c). A surrounding white circular “guide line” aided stable fixation of the patterns presented to the two eyes. Top and left nonius lines were presented with the guideline to the left eye, and bottom and right nonius lines were presented to the right eye. The two eyes were centered on the same location in the visual field by perceptual alignment of the horizontal and vertical nonius lines. Guidelines and nonius lines were metameric to EES. A 0.1 deg gap between the center and the surround was introduced so observers could easily distinguish the central rivalrous targets from the surrounds (as in Fig. 1c).
In the first experiment, the rivalrous centers were uniform 1-deg circular disks with different chromaticities (four different pairs of chromaticities were used; Fig. 1a). The surround was either uniform or a 4 cycle per degree (cpd) square-wave grating, with or without luminance contrast. The surround was the same in the two eyes. The chromaticity of the surround was the same as the chromaticity of one of the rivalrous targets. In separate runs, the chromaticity of the surround was counterbalanced for the two chromaticities in the pair. The luminance of the chromatic regions was fixed at 8 cd/m2. The luminance of the achromatic regions in the gratings was virtually 0 (black) or 8 cd/m2, forming conditions referred to as a chromatic/black (luminance-contrast) grating or a chromatic/EES (equiluminant) grating, respectively.
To test whether the influence of a chromatic surround depended on the relative orientation of the center and surrounding gratings, a second experiment used 4 cpd square-wave gratings in both center and surround (Fig. 1c). The color-rivalrous central grating always was vertical; the surrounding grating was varied in orientation (vertical or horizontal) and luminance contrast (chromatic/black or equiluminant chromatic/EES).
Procedure
Within a session, all four pairs of chromaticities were tested twice with the stimuli switched between the two eyes. This controlled for the eye dominance of individual observers. When a session began, the achromatic (EES) guidelines and nonius lines were presented with an EES uniform field in the circular aperture where rivalrous targets were subsequently presented. Observers started the experiment by pressing a pre-assigned button to report fusion of the guidelines and alignment of the nonius lines. Then, the left stimulus alone was presented for 5 sec followed by the right stimulus alone for 5 sec. When observers had confidently identified each stimulus, so they could identify a dominant stimulus from either eye, they again pressed the pre-assigned button. Following 30 sec of dark adaptation, the central rivalrous targets and their surrounds were presented for 30 sec.
Perceptual alternation was measured during dichoptic presentation of the two rivalrous stimuli. Observers used a game pad to report their percept by pressing separate buttons. For example, when the complete central stimulus presented to the left eye was perceived, observers held a button until the percept changed. When the complete central stimulus presented to the right eye was perceived, observers held a different button. An additional button was assigned for the percept of binocular color mixture. Observers were instructed not to press any button for other percepts such as a patch-like percept. Each experiment was repeated three times, always on a different day. The three replicated measurements for each condition were used for analysis.
The total duration for each percept, called the duration of exclusive visibility (Hollins, 1980), was used for analysis, as in recent studies measuring predominance during binocular rivalry (Blake, Yu, Lokey & Norman, 1998; Andrews & Blakemore, 2002; Paffen, Tadin, te Pas, Blake & Verstraten, 2006). An alternative metric known as mean dominance duration (MDD) is posited to index the “strength” of rivalrous stimuli, according to Levelt's (1965) second proposition, but the relation between MDD and the strength of rivalrous stimuli is controversial (Bossink, Talmeier & De Weert, 1993; Brascamp, van Ee, Noest, Jacobs & van den Berg, 2006).
Observers
Author S.W.H. (male) and university students M.K. (female) and M.O. (female), who were naïve about the purpose of the study, participated in all experiments. Each was screened for color vision defects using a Nagel Anomaloscope. An equiluminant level of each CRT phosphor was measured for every observer using heterochromatic modulation photometry at 15 Hz (Pokorny, Smith & Lutze, 1989). This excluded luminance artifacts due to individual differences. S-cone isolation for each observer was verified using the minimally distinct border method (Tansley & Boynton, 1978).
Results
The influence of chromatic context on binocular color rivalry
The influence of surround chromaticity on the duration of predominance during binocular color rivalry was measured by varying the chromaticity of the center and the surround. The measurements showed that the duration of exclusive visibility for each rivalrous target was affected by the surround chromaticity.
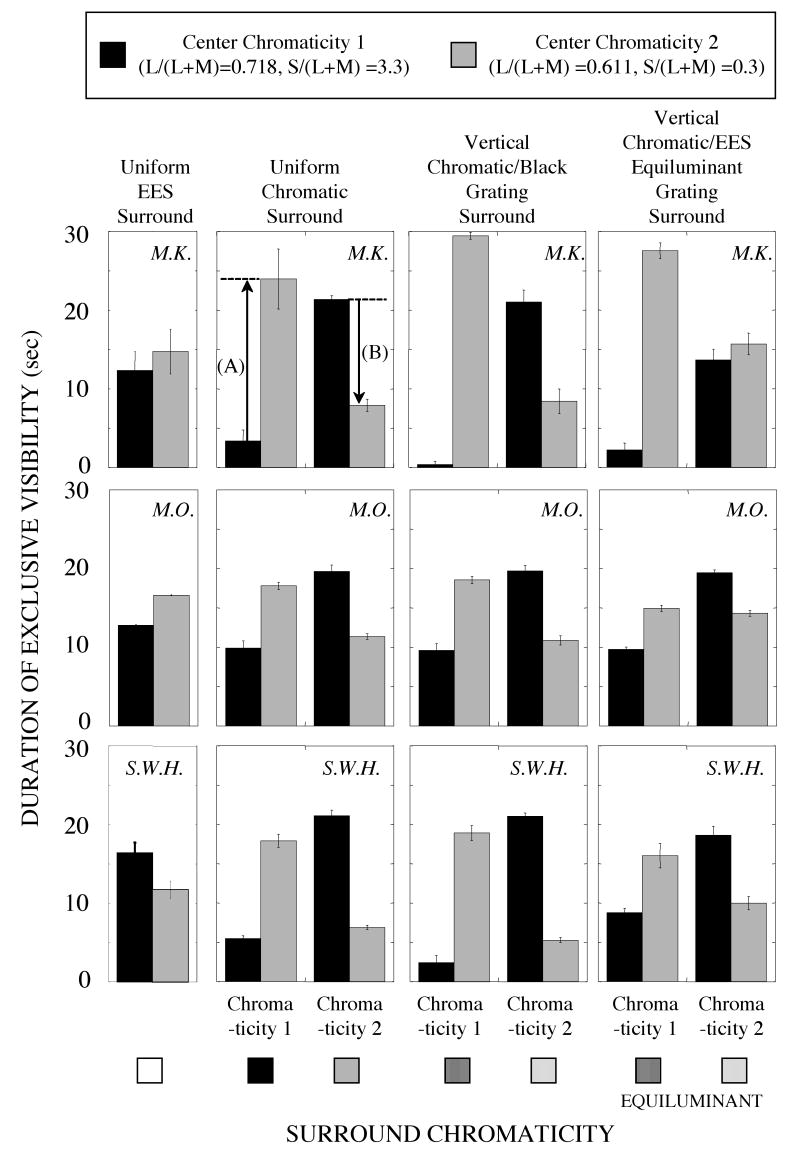
Results from three observers with one chromaticity pair are shown in Fig. 2 (chromaticity 1: L/(L+M)=0.718, S/(L+M)=3.3, chromaticity 2: L/(L+M)=0.611, S/(L+M)=0.3). Each row shows results from one observer. The duration of exclusive visibility of each central chromaticity during 30 seconds of dichoptic presentation is shown on the vertical axis. The type of surround is on the horizontal axis. The values within each panel do not sum to 30 seconds because the percept of binocular color mixture, which is not shown in the figure, was sometimes observed. The first column shows results when the surround was a uniform EES field. This was the baseline condition to which the influence of other surrounds was compared. The second column from the left shows results with uniform chromatic surrounds at the chromaticity of one of the centers. In the third column, the surround was a 4 cpd vertical grating with 100% luminance contrast; in the fourth column, the surround was an equiluminant 4 cpd vertical grating.
Figure 2.
The chromatic surround's influence on duration of exclusive visibility with uniform chromatically rivalrous central targets, as in Fig. 1b, for chromaticity pair 1 (L/(L+M)=0.718, S/(L+M)=3.3 and L/(L+M)=0.611, S/(L+M)=0.3). Each column is for a different type of surround. The arrows labeled A and B in the second panel of the top row show the components used to calculate the chromatic Surround Influence (see text). Note that A is positive and B is negative in this example.
All three observers showed a strong chromatic-surround influence on the duration of exclusive visibility. When the surround had chromaticity 1, the duration of predominance for chromaticity 1 decreased and the duration of predominance for chromaticity 2 increased, compared to the condition when the surround was an EES uniform field. Similarly, when the surround had chromaticity 2, the duration of predominance for chromaticity 1 increased and the duration of chromaticity 2 decreased. These results were consistent across the other chromaticity pairs shown in Fig. 1a: A three-way analysis of variance (center chromaticity × surround chromaticity × surround condition), done separately for each chromaticity pair and observer, showed a significant interaction between the surround chromaticity and the center chromaticity for every observer and chromaticity pair (each of the 12 values of F(1,24) exceeded 100, P<0.001).
The magnitude of the chromatic surround's influence was quantified by comparing the difference in predominance time with one surround chromaticity to the difference in predominance time with the other surround chromaticity of the chromaticity pair. The difference in the duration of predominance (center chromaticity 2 – center chromaticity 1) with surround chromaticity 1 [2] is represented by the arrow A [arrow B] in Fig. 2 (second panel, top row). Note that A is positive and B is negative. The Surround Influence is calculated by subtracting B from A, and then dividing by 2 [that is, (A – B)/2].
The magnitude of the chromatic Surround Influence is shown in Fig. 3 for all four pairs of chromaticities (see legend) and for all three observers. The horizontal axis shows the surround configuration. The error bars represent the standard error of the mean of 3 measurements from separate days. The four differently shaded bars represent the four chromaticity pairs. All values are positive, which shows that the predominance of chromaticity in the center is affected by the chromaticity in the surround.
Figure 3.
The magnitude of the chromatic Surround Influence (vertical axis). Rivalrous centers were uniform disks. The horizontal axis indicates the type of surround. Differently shaded bars represent different chromaticity pairs (see legend). Each panel shows results from one observer.
A planned orthogonal contrast tested whether the uniform and vertical chromatic/black-grating surrounds (100% luminance contrast), on average, had greater Surround Influence than the vertical equiluminant chromatic/EES surround. This was the case for every chromaticity pair and observer (the 12 values of F(1,24) ranged from 5.36 to 45.21, P<0.05).
Another planned contrast examined the difference in the chromatic Surround Influence between the uniform surround and the vertical chromatic/black-grating surround. While five of 12 tests were significant (none for observer M.K.; chromaticity pairs 2, 3 and 4 for observer M.O.; and chromaticity pairs 1 and 3 for observer S.W.H.), the direction of the difference was not consistent (greater influence of the uniform surround in 2 instances, and greater influence of the grating surround in 3 instances).
The influence of orientation of chromatic context on binocular color rivalry
The influence of the chromatic surround's orientation was measured as in the previous experiment except that the rivalrous central targets were 4 cpd vertical gratings with 100% luminance contrast rather than uniform disks (Fig. 1c). The measurements showed that the duration of exclusive visibility for each rivalrous target depended on the chromaticity of the surround, as before, but there was no significant effect of surround orientation.
The magnitude of the chromatic Surround Influence on the color-rivalrous central vertical gratings is shown in Fig. 4. The four surround conditions are on the horizontal axis. The differently shaded bars represent different chromaticity pairs (see legend). Negative values occurred in some conditions but none was significantly different from zero.
Figure 4.
As Fig. 3 but with surrounding gratings that were varied in orientation and with rivalrous centers that were vertical gratings.
A planned orthogonal contrast showed that the magnitude of surround influence from chromatic/black-grating surrounds was larger than from equiluminant grating surrounds, for every chromaticity pair and observer (the smallest of the 12 values of F(1,40) was 5.28, P<0.05). This is consistent with the previous experiment. A second planned orthogonal contrast tested the null hypothesis that the surround influence from the vertical chromatic/black-grating surround was equal to that from the horizontal chromatic/black-grating surround. Recall the central test grating always was vertical. This null hypothesis could not be rejected for any chromaticity pair for any observer (the largest of the 12 values of F(1,40) was 2.60, P>0.10).
Discussion
The influence of non-rivalrous chromatic context on binocular color rivalry of a central target was measured for various chromatic and spatial configurations. There were two main findings. First, the chromatic-surround influence was stronger with a surround that was a uniform field or a grating with luminance contrast (chromatic/black grating) than with an equiluminant grating (chromatic/EES grating). Second, there was no significant effect on color rivalry due to the orientation of the surrounding grating. When the color-rivalrous central targets were vertical gratings, vertically and horizontally oriented surrounds never differed significantly for any observer with any chromaticity pair (12 conditions, none of which showed a statistically reliable effect of orientation).
Theoretical underpinning for the chromatic-surround influence on binocular color rivalry
A stronger influence from uniform and chromatic/black-grating surrounds than from equiluminant chromatic/EES-grating surrounds implicates a color selective neural response that is greater with a chromatic/black grating or a chromatic uniform field than with an equiluminant chromatic/EES grating. Consider three distinct types of chromatically selective receptive fields found in early visual cortex (V1 and V2). One has a low-pass spatial characteristic. The other two are spatially bandpass: simple spatial antagonism and double-opponency (Johnson, Hawken & Shapley, 2001, Conway, 2001; see Fig. 5).
Figure 5.
Response properties of three types of receptive field. The horizontal axis in each panel is the spatial frequency of the stimulus and the vertical axis is the normalized response of each receptive-field type. The solid line represents the response to a chromatic/black grating; the dashed line represents the response to an equiluminant chromatic/ EES grating (sometimes overlapping the solid line and not visible). The open circle shows the response to a uniform chromatic field. The dark horizontal line at 0.0 in each panel shows the response to a uniform EES field. (a) The response properties of a chromatically selective receptive field without spatial antagonism (the solid and dashed lines overlap). (b) The response properties of a chromatically selective and spatially antagonistic receptive field. (c) The response properties of a double-opponent receptive field (the solid and dashed lines overlap).
The response properties of these cell types can be evaluated by modeling. Here, the receptive field was constructed by combining a spatially Gaussian response to L-cone stimulation and a separate Gaussian response to M-cone stimulation. The relative size of each Gaussian was determined according to the posited receptive-field properties. For a low-pass receptive field, the widths of the two Gaussians were identical. For a bandpass receptive field, the width of the spatially antagonistic surround was set to twice that of the center with the overall width set for peak sensitivity near 2 cpd, as suggested by Johnson, Hawken and Shapley (2001). Another type of bandpass receptive field - double opponency - was modeled by combining two band-pass receptive fields having opposite L- and M-cone polarities. For each receptive field, the relative L-cone to M-cone response was scaled to give a null response to equal-energy-spectrum ‘white’.
The response of each type of receptive field was determined for the various surrounds used in the experiments. The linking assumption relating the model to the measurements is that the stronger the response from the receptive field, the stronger the Surround Influence on color rivalry. Sine-wave grating stimuli were used to calculate receptive field responses. Contributions are negligible from the higher harmonics of the 4 cpd square-wave grating used in experiments here, for both equiluminant and chromatic/black luminance gratings.
Consider a sine-wave grating of a single chromaticity (L/(L+M)=0.718, S/(L+M)=1.0) varied in luminance (‘chromatic/black grating’; Michelson contrast 100%). Spatial frequency was varied from 0.2 to 10 cpd. A second stimulus was an equiluminant sine-wave grating (‘chromatic/EES grating’) with fixed luminance equal to the peak of the chromatic/black grating. Note that the space-average luminance of this equiluminant chromatic/EES grating was twice that of the chromatic/black grating, as in the experimental conditions. The mean chromaticity of the chromatic/EES grating was L/(L+M)=0.692, S/(L+M)=1.0, with the L/(L+M) value varied in sine-wave fashion between 0.718 and 0.667 (EES). The third stimulus was a uniform chromatic field (L/(L+M)=0.718, S/(L+M)=1.0) with luminance equal to the peak of the chromatic/black grating, which is the uniform stimulus used in experiments. A uniform EES field served as baseline, for which the response was zero by design because cells with balanced opponent input from L and M cones, as assumed here, do not respond to a uniform EES field.
The responses to these stimuli are shown in Fig. 5. Each panel shows responses for one type of receptive field described above. The horizontal axis is the spatial frequency of the grating (log scale) and the vertical axis is the normalized response level. For each type of receptive field, responses were normalized to the peak response over all the stimuli. The responses to the chromatic/black and chromatic/EES gratings are shown by solid and dashed lines, respectively (though sometimes they are overlapping and indistinguishable), while the responses to the uniform chromatic field are shown by open circles. The response to the uniform EES field (baseline condition) is indicated by the dark horizontal line at 0.0 in each panel.
Spatially non-antagonistic receptive field
Cells with this type of receptive field have a low-pass response to all chromatic stimuli, equiluminant or not (Fig. 5a, solid and dashed lines overlap). The response decreases rapidly as the spatial frequency of the stimulus increases, with the largest response from a uniform chromatic field. The response to a chromatic-black grating is the same as the response to a chromatic/EES grating at all spatial frequency because the space-average luminance of the chromatic/black grating was half of the space-average luminance of the chromatic/EES grating, as in the experiments.
Spatially antagonistic receptive field
Cells with chromatically opponent center and surround are bandpass for chromatic/black gratings and low-pass for chromatic/EES gratings (Fig. 5b, solid and dashed lines). This type of receptive field has a weaker response to a chromatic/EES grating than to a chromatic/black grating. Note that the response to a uniform chromatic field (open circle) has a response similar to that of a chromatic/black grating of about 3-4 cpd (4 cpd was used in experiments here).
Double-opponent receptive field
Double-opponent cells do not respond to a uniform chromatic stimulus because the excitatory center and inhibitory surround are stimulated equally by a uniform stimulus covering the receptive field (Fig. 5c, indicated by the open circle on the horizontal line at 0.0). There is a bandpass response to chromatic gratings, equiluminant or not (solid and dashed lines overlap in Fig. 5c).
Among these three types of receptive field, only center-surround spatial antagonism is consistent with the characteristics of the chromatic Surround Influence revealed by the experiments: 1) a 4 cpd chromatic/black grating had a stronger Surround Influence than a chromatic/EES grating at the same spatial frequency, and 2) the Surround Influence was similar for a uniform chromatic field and a chromatic/black grating at 4 cpd. All three types of receptive fields respond to chromatic stimuli, but only the center-surround, single-opponent receptive field responds more strongly to a chromatic/black grating than to an equiluminant chromatic/EES grating. The modeling, therefore, suggests that the response from this type of color-selective receptive field mediates the influence of chromatic context on binocular color rivalry.
The L/M receptive fields in Fig. 5 cannot, of course, account for the results with the pure S-cone chromaticity pair (Pair 4). Ganglion cells respond to S-cone stimulation but do not have center-surround spatial antagonism (Dacey, 1996); such cells are low-pass for pure S-cone stimulation. As shown above, a low-pass receptive field is not consistent with the experimental results, which show a consistent chromatic Surround Influence with the pure S-cone chromaticity pair (Figs. 3 and 4). This chromatic Surround Influence may be partially explained by an S-cone-specific center-surround receptive field, which accounts for color shifts from patterned backgrounds (Monnier & Shevell, 2003, 2004). This type of receptive field responds more strongly to a chromatic/black grating than to a chromatic/EES grating, though it cannot explain the results due to varying only S-cone stimulation in a uniform surround because the receptive-field has a null response to any uniform field when the center and surround have balanced weights.
Separate processing of color and form
The separate processing hypothesis posits that different aspects of a visual stimulus, such as color, form, and motion, are encoded within separate visual processing streams (Livingstone & Hubel, 1988; Felleman & Van Essen, 1991; Zeki, 1993). According to the strong form of the hypothesis, chromatic information is processed by cells sensitive to chromaticity differences but not orientation differences. Separate processing of color and form, however, is often challenged by studies both physiological (Leventhal, Thompson, Liu, Zhou & Ault, 1995; Gegenfurtner, Kiper & Fenstemaker, 1996; Kiper, Fenstemaker & Gegenfurtner, 1997; Friedman, Zhou & von der Heydt, 2003; Johnson, Hawken & Shapley, 2001, 2004) and psychophysical (Clifford, Pearson, Forte & Spehar, 2003; Clifford, Spehar, Solomon, Martin & Zaidi, 2003). If orientation and color are inseparably encoded, a surround's influence on color should depend on the orientation of the surround.
Separate processing of color and form is supported by the results showing no significant difference (among any of 12 tests) in the magnitude of Surround Influence from the vertical versus horizontal grating-surrounds, when the color rivalrous centers were vertical gratings. Multi-selective cells in V1 and V2 that are tuned to the conjunction of orientation and color (Johnson et al., 2001, 2004; Friedman et al., 2003) allow the possibility that color and spatial form may be processed in a combined manner early in visual cortex. Resolution of rivalry is related to activity of cells in V1 and V2 (Tong & Engel, 2001; Lee & Blake, 2002). If color and form are inseparable, binocular color rivalry should be affected by the spatial structure of the stimuli. In this case, the chromatic-surround influence should be weaker when the orientation of the central and surrounding gratings are perpendicular to each other, compared to when the orientations are identical. This was not found here.
The chromaticity of a surrounding grating does affect the perceived orientation of a grating in the center (Clifford, Pearson, Forte & Spehar, 2003; Clifford, Spehar, Solomon, Martin & Zaidi, 2003). This shows that cells tuned to both orientation and color contribute to a surround's influence on perceived orientation. The same has not been shown for color perception. While being mindful of a Type II error, the consistent failure to find a significant effect of spatial structure on the chromatic Surround Influence supports the view that color and form are not inseparably represented within a single neural pathway.
An unsolved question is how color is processed in visual cortex. Do multi-selective cells contribute to the perception of hue? The results here do not exclude it. A cell tuned to both an orientation and chromaticity may contribute to both perceived orientation and color, but there is an implicit ambiguity in the response from a single cell. A decrease in the cell's response to an optimal orientation and chromaticity may be due to a change in only chromaticity or only orientation. These two possibilities (and infinitely many others) cannot be distinguished from the response of one cell alone. A single multi-selective cell cannot be the neural substrate for color perception. If multi-selective cells contribute to color perception, they must be part of an ensemble of responses.
Acknowledgments
This research was supported by NIH grant EY-04802. Publication supported in part by an unrestricted grant to the Department of Ophthalmology & Visual Science from Research to Prevent Blindness.
Footnotes
Judd (1951) chromaticity coordinates (x, y) were: Pair 1 (0.30, 0.17) and (0.28, 0.56); Pair 2 (0.48, 0.40) and (0.21, 0.19); Pair 3 (0.40, 0.30) and (0.25, 0.38); Pair 4 (0.26, 0.17) and (0.40, 0.46).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alais D, Blake R. Grouping visual features during binocular rivalry. Vision Research. 1999;39:4341–4345. doi: 10.1016/s0042-6989(99)00146-7. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Blakemore C. Integration of motion information during binocular rivalry. Vision Research. 2002;42:301–3091. doi: 10.1016/s0042-6989(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nature Review Neuroscience. 2002;3:1–11. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Blake R, Sobel KV, Gilroy LA. Visual motion retards alternations between conflicting perceptual interpretations. Neuron. 2003;39:869–878. doi: 10.1016/s0896-6273(03)00495-1. [DOI] [PubMed] [Google Scholar]
- Blake R, Yu K, Lokey M, Norman H. Binocular rivalry and motion perception. Journal of Cognitive Neuroscience. 1998;10:46–60. doi: 10.1162/089892998563770. [DOI] [PubMed] [Google Scholar]
- Bossink CJH, Stalmeier PFM, de Weert CMM. A test of Levelt's second proposition for binocular rivalry. Vision Research. 1993;33:1413–1419. doi: 10.1016/0042-6989(93)90047-z. [DOI] [PubMed] [Google Scholar]
- Brascamp JW, van Ee R, Noest AJ, Jacobs RHAH, van den Berg AV. The time course of binocular rivalry reveals a fundamental role of noise. Journal of Vision. 2006;6:1244–1256. doi: 10.1167/6.11.8. [DOI] [PubMed] [Google Scholar]
- Carter OL, Campbell TG, Liu GB, Wallis G. Contradictory influence of context on predominance during binocular rivalry. Clinical and Experimental Optometry. 2004;87:153–152. doi: 10.1111/j.1444-0938.2004.tb03168.x. [DOI] [PubMed] [Google Scholar]
- Clifford CWG, Pearson J, Forte JD, Spehar B. Colour and luminance selectivity of spatial and temporal interactions in orientation perception. Vision Research. 2003;43:2885–2893. doi: 10.1016/j.visres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Clifford CWG, Spehar B, Solomon SG, Martin PR, Zaidi Q. Interactions between color and luminance in the perception of orientation. Journal of Vision. 2003;3:106–115. doi: 10.1167/3.2.1. [DOI] [PubMed] [Google Scholar]
- Chong SC, Blake R. Exogenous attention and endogenous attention influence initial dominance in binocular rivalry. Vision Research. 2006;46:1794–1803. doi: 10.1016/j.visres.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Conway BR. Spatial structure of cone inputs to color cells in alert macaque primary visual cortex (V-1) Journal of Neuroscience. 2001;21:2768–2783. doi: 10.1523/JNEUROSCI.21-08-02768.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Circuitry for color coding in the primate retina. Proceedings of the National Academy of Sciences USA. 1996;93:582–588. doi: 10.1073/pnas.93.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Zhou H, von der Heydt R. The coding of uniform colour figures in monkey visual cortex. Journal of Physiology. 2003;548:593–613. doi: 10.1113/jphysiol.2002.033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Blake R. Spatial interactions in binocular rivalry. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:362–370. doi: 10.1037//0096-1523.18.2.362. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC, Fenstemaker SB. Processing of color, form, and motion in macaque area V2. Visual Neuroscience. 1996;13:161–172. doi: 10.1017/s0952523800007203. [DOI] [PubMed] [Google Scholar]
- Hollins M. The effect of contrast on the completeness of binocular rivalry suppression. Perception and Psychophysics. 1980;27:550–556. doi: 10.3758/bf03198684. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sagawa K. Binocular color fusion limit. Journal of the Optical Society America. 1979;69:316–321. doi: 10.1364/josa.69.000316. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nature Neuroscience. 2001;4:409–416. doi: 10.1038/86061. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Hawken MJ, Shapley R. Cone inputs in macaque primary visual cortex. Journal of Neurophysiology. 2004;91:2501–2514. doi: 10.1152/jn.01043.2003. [DOI] [PubMed] [Google Scholar]
- Judd DB. Report of US Secretariat, Committee on Colorimetry and Artificial Daylight. Proceedings of the CIE 1; Stockholm. Paris: Bureau Central CIE; 1951. p. 11. [Google Scholar]
- Kiper DC, Fenstemaker SB, Gegenfurtner KR. Chromatic properties of neurons in macaque area V2. Visual Neuroscience. 1997;14:1061–1072. doi: 10.1017/s0952523800011779. [DOI] [PubMed] [Google Scholar]
- Kovács I, Papathomas TV, Yang M, Fehér Á. When the brain changes its mind: interocular grouping during binocular rivalry. Proceedings of the National Academy of Sciences USA. 1996;93:15508–15511. doi: 10.1073/pnas.93.26.15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Blake R. V1 activity is reduced during binocular rivalry. Journal of Vision. 2002;2:618–626. doi: 10.1167/2.9.4. [DOI] [PubMed] [Google Scholar]
- Levelt wJM. On binocular rivalry. Soesterberg: Institute for Perception RVO-TNO; 1965. [Google Scholar]
- Leventhal AG, Thompson KG, Liu D, Zhou Y, Ault SJ. Concomitant sensitivity to orientation, direction, and color of cells in layers 2, 3, and 4 of monkey striate cortex. Journal of Neuroscience. 1995;15:1808–1818. doi: 10.1523/JNEUROSCI.15-03-01808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- MacLeod DIA, Boynton RM. Chromaticity diagram showing cone excitation by stimuli of equal luminance. Journal of the Optical Society of America. 1979;69:1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- Monnier P, Shevell SK. Large shifts in color appearance from patterned chromatic backgrounds. Nature Neuroscience. 2003;6:801–802. doi: 10.1038/nn1099. [DOI] [PubMed] [Google Scholar]
- Monnier P, Shevell SK. Chromatic induction from S-cone pattern. Vision Research. 2004;44:849–856. doi: 10.1016/j.visres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Paffen CLE, Alais D, Verstraten FAJ. Center-surround inhibition deepens binocular rivalry suppression. Vision Research. 2005;45:2642–2649. doi: 10.1016/j.visres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Paffen CLE, Tadin D, te Pas SF, Blake R, Verstraten FAJ. Adaptive center-surround interactions in human vision revealed during binocular rivalry. Vision Research. 2006;46:599–604. doi: 10.1016/j.visres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Paffen CLE, te Pas SF, Kanai R, van der Smagt MJ, Verstraten FAJ. Center-surround interactions in visual motion processing during binocular rivalry. Vision Research. 2004;44:1635–1639. doi: 10.1016/j.visres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Smith VC, Lutze M. Heterochromatic modulation photometry. Journal of Optical Society America A. 1989;6:1618–1623. doi: 10.1364/josaa.6.001618. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Peirce JW, Lennie P. The impact of suppressive surrounds on chromatic properties of cortical neurons. Journal of Neuroscience. 2004;24:148–160. doi: 10.1523/JNEUROSCI.3036-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansley BW, Boynton RM. Chromatic border perception: the role of red- and green-sensitive cones. Vision Research. 1978;18:683–697. doi: 10.1016/0042-6989(78)90147-5. [DOI] [PubMed] [Google Scholar]
- Tong F, Engel SA. Interocular rivalry revealed in the human cortical blind-spot representation. Nature. 2001;411:195–199. doi: 10.1038/35075583. [DOI] [PubMed] [Google Scholar]
- Zeki S. A Vision of the Brain. Blackwell; Oxford: 1993. [Google Scholar]