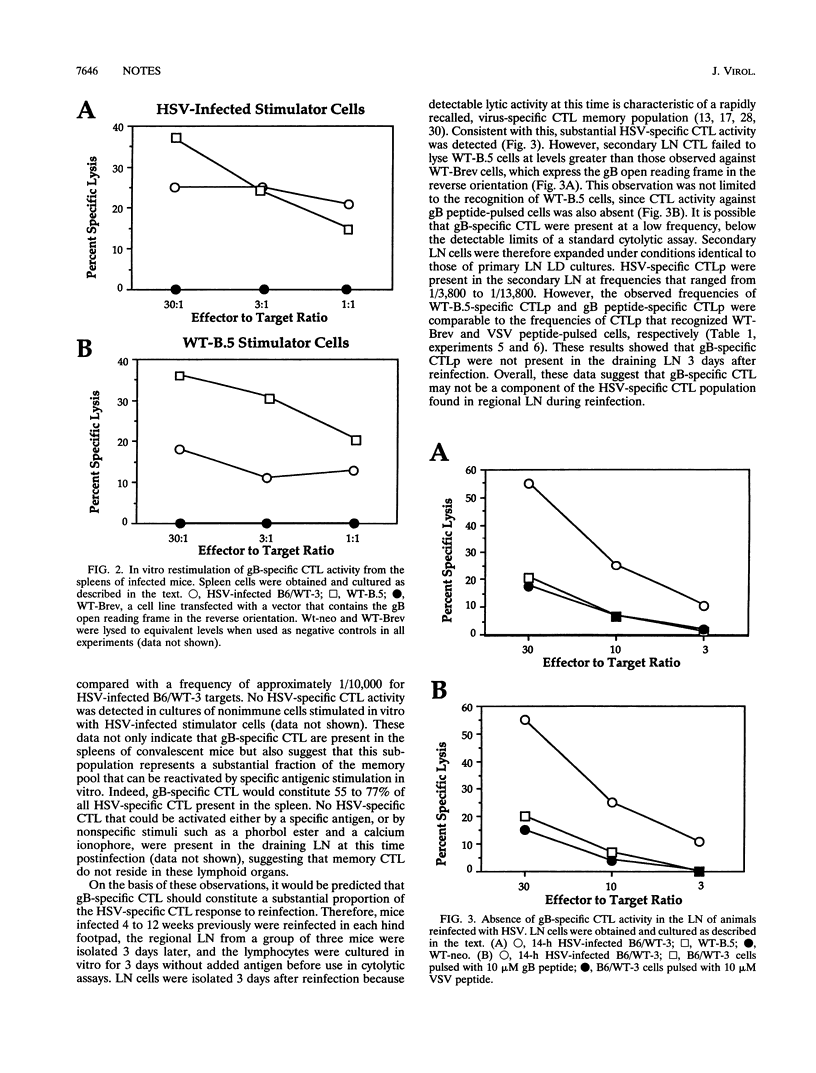
Abstract
The immune response to herpes simplex virus type 1 (HSV-1) infection in C57BL/6 mice includes a population of major histocompatibility complex class I-restricted cytolytic T lymphocytes (CTL) that recognize the structural glycoprotein gB. To gain insight into the importance of this CTL subpopulation in vivo, gB-specific CTL present in the regional lymph nodes after a primary infection and after a reinfection of convalescent animals were analyzed. In a primary infection, gB-specific CTL precursors (CTLp) that recognized either a cell line constitutively expressing gB or cells pulsed with the optimal Kb-restricted gB epitope 498SSIEFARL505 were present at an estimated frequency of 1/12,000 compared with a frequency of 1/3,000 for CTLp which recognized cells infected with HSV-1 itself. In convalescent mice responding to reinfection, HSV-specific CTLp were present at an estimated frequency of 1/4,000 to 1/14,000. However, gB-specific CTLp could not be detected at this site. These findings suggest that CTL specific for an immunodominant epitope contribute substantially to the primary response but may not be a component of the HSV-specific CTL population that responds rapidly to reinfection in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R. Immunological memory against viruses. Semin Immunol. 1992 Apr;4(2):105–109. [PubMed] [Google Scholar]
- Bonneau R. H., Fu T. M., Tevethia S. S. In vivo priming and activation of memory cytotoxic T-lymphocytes (CTL) by a chimeric simian virus 40 T antigen expressing an eight amino acid residue herpes simplex virus gB CTL epitope. Virology. 1993 Dec;197(2):782–787. doi: 10.1006/viro.1993.1657. [DOI] [PubMed] [Google Scholar]
- Bonneau R. H., Jennings S. R. Herpes simplex virus-specific cytolytic T lymphocytes restricted to a normally low responder H-2 allele are protective in vivo. Virology. 1990 Feb;174(2):599–604. doi: 10.1016/0042-6822(90)90113-6. [DOI] [PubMed] [Google Scholar]
- Bonneau R. H., Jennings S. R. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J Virol. 1989 Mar;63(3):1480–1484. doi: 10.1128/jvi.63.3.1480-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau R. H., Salvucci L. A., Johnson D. C., Tevethia S. S. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993 Jul;195(1):62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Cantin E. M., Eberle R., Baldick J. L., Moss B., Willey D. E., Notkins A. L., Openshaw H. Expression of herpes simplex virus 1 glycoprotein B by a recombinant vaccinia virus and protection of mice against lethal herpes simplex virus 1 infection. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5908–5912. doi: 10.1073/pnas.84.16.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter V. C., Schaffer P. A., Tevethia S. S. The involvement of herpes simplex virus type 1 glycoproteins in cell-mediated immunity. J Immunol. 1981 May;126(5):1655–1660. [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen K. D. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993 Jun;91(6):2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Val M., Münch K., Reddehase M. J., Koszinowski U. H. Presentation of CMV immediate-early antigen to cytolytic T lymphocytes is selectively prevented by viral genes expressed in the early phase. Cell. 1989 Jul 28;58(2):305–315. doi: 10.1016/0092-8674(89)90845-3. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Allan W., Eichelberger M., Carding S. R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- Effros R. B., Bennink J., Doherty P. C. Characteristics of secondary cytotoxic T-cell responses in mice infected with influenza A viruses. Cell Immunol. 1978 Mar 15;36(2):345–353. doi: 10.1016/0008-8749(78)90278-2. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Gallichan W. S., Johnson D. C., Graham F. L., Rosenthal K. L. Mucosal immunity and protection after intranasal immunization with recombinant adenovirus expressing herpes simplex virus glycoprotein B. J Infect Dis. 1993 Sep;168(3):622–629. doi: 10.1093/infdis/168.3.622. [DOI] [PubMed] [Google Scholar]
- Hanke T., Graham F. L., Rosenthal K. L., Johnson D. C. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991 Mar;65(3):1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. B., Blanden R. V., Parrish C. R., Müllbacher A. Restimulated memory Tc cells have a higher apparent avidity of interaction with targets than primary virus-immune Tc cells as indicated by anti-CD8 blocking. Immunol Cell Biol. 1992 Aug;70(Pt 4):259–265. doi: 10.1038/icb.1992.33. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Bonneau R. H., Smith P. M., Wolcott R. M., Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991 Mar;133(1):234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Pan S., Knowles B. B., Tevethia S. S. Recognition of herpes simplex virus antigens on the surface of mouse cells of the H-2b haplotype by virus-specific cytotoxic T lymphocytes. J Immunol. 1984 Jan;132(1):475–481. [PubMed] [Google Scholar]
- Karupiah G., Xie Q. W., Buller R. M., Nathan C., Duarte C., MacMicking J. D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993 Sep 10;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C. R. Immunological memory. Adv Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- Martin S., Mercadal C. M., Weir J. P., Rouse B. T. The proportion of herpes simplex virus-specific cytotoxic T lymphocytes (Tc) that recognize glycoprotein C varies between individual mice and is dependent on the form of immunization. Viral Immunol. 1993 Spring;6(1):21–33. doi: 10.1089/vim.1993.6.21. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Goldsmith C. H., Rosenthal K. L., Brais L. J. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J Infect Dis. 1989 Mar;159(3):460–466. doi: 10.1093/infdis/159.3.460. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Graham F. L., Hanke T., Johnson D. C. Protection of mice against lethal challenge with herpes simplex virus by vaccination with an adenovirus vector expressing HSV glycoprotein B. Virology. 1989 Mar;169(1):244–247. doi: 10.1016/0042-6822(89)90064-0. [DOI] [PubMed] [Google Scholar]
- Müllbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994 Jan 1;179(1):317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Quartey-Papafio R., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: functional analysis of lymph node cells during periods of acute and latent infection, with reference to cytotoxic and memory cells. J Gen Virol. 1980 Aug;49(2):309–317. doi: 10.1099/0022-1317-49-2-309. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Momburg F. Cell biology of antigen presentation. Curr Opin Immunol. 1993 Feb;5(1):27–34. doi: 10.1016/0952-7915(93)90077-6. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Starzinski-Powitz A., Röllinghoff M., Falks D., Wagner H. T-cell-mediated cytotoxicity against herpes simplex virus-infected target cells. Nature. 1977 Feb 17;265(5595):630–632. doi: 10.1038/265630a0. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Pretell J., Greenfield R. S., Tevethia S. S. Biology of simian virus 40 (SV40) transplantation antigen (TrAg). V In vitro demonstration of SV40 TrAg in SV40 infected nonpermissive mouse cells by the lymphocyte mediated cytotoxicity assay. Virology. 1979 Aug;97(1):32–41. doi: 10.1016/0042-6822(79)90370-2. [DOI] [PubMed] [Google Scholar]
- Schmid D. S., Rouse B. T. The role of T cell immunity in control of herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:57–74. doi: 10.1007/978-3-642-77247-4_4. [DOI] [PubMed] [Google Scholar]
- Seid J. M., Liberto M., Bonina L., Leung K. N., Nash A. A. T cell-macrophage interactions in the immune response to herpes simplex virus: the significance of interferon-gamma. J Gen Virol. 1986 Dec;67(Pt 12):2799–2802. doi: 10.1099/0022-1317-67-12-2799. [DOI] [PubMed] [Google Scholar]
- Simmons A., Tscharke D., Speck P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- Smith P. M., Wolcott R. M., Chervenak R., Jennings S. R. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology. 1994 Jul;202(1):76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tevethia M. J., Kalderon D., Smith A. E., Tevethia S. S. Clustering of antigenic sites recognized by cytotoxic T lymphocyte clones in the amino terminal half of SV40 T antigen. Virology. 1988 Feb;162(2):427–436. doi: 10.1016/0042-6822(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Witmer L. A., Rosenthal K. L., Graham F. L., Friedman H. M., Yee A., Johnson D. C. Cytotoxic T lymphocytes specific for herpes simplex virus (HSV) studied using adenovirus vectors expressing HSV glycoproteins. J Gen Virol. 1990 Feb;71(Pt 2):387–396. doi: 10.1099/0022-1317-71-2-387. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]



