Abstract
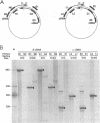
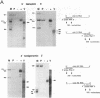
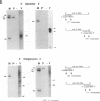
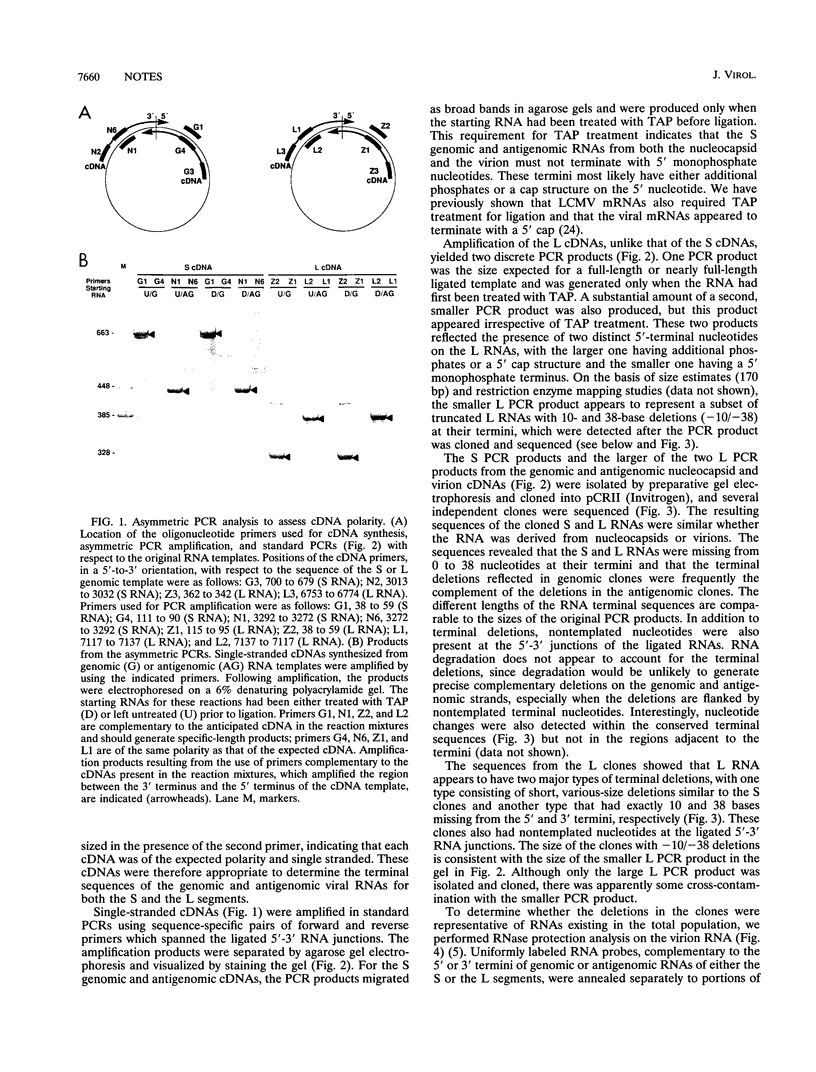
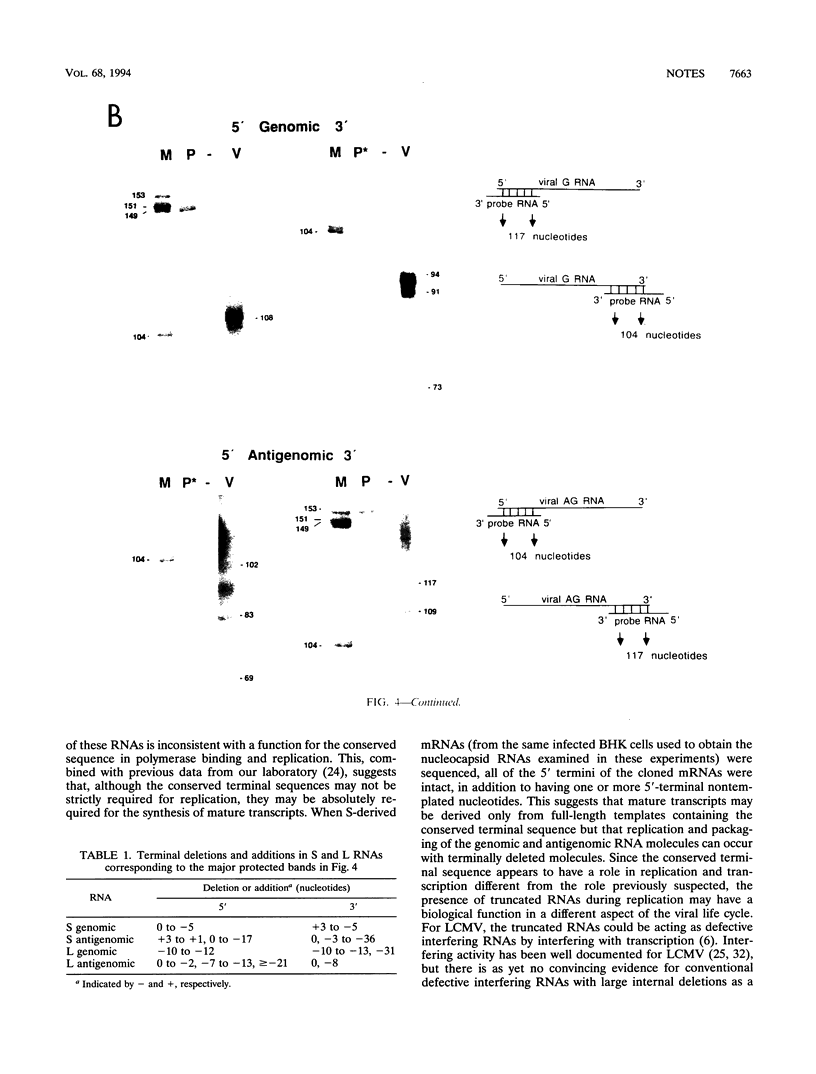
Sequence analysis of lymphocytic choriomeningitis virus L and S RNAs has revealed evidence of heterogeneity within the termini of the genomic and antigenomic RNAs. The RNAs are missing from 0 to 38 bases, show characteristic patterns of deleted nucleotides at both 5' and 3' termini, and often have a nontemplated base at the terminus. The same deletions, at either the 5' or the 3' terminus of the genomic L and S RNAs, are frequently found in the complementary strand of antigenomic RNA, suggesting that RNAs with deleted termini may be recognized as functional templates for replication. RNAs extracted from virions, or viral nucleocapsids isolated from acutely infected cells, are similar in the nature and extent of terminal heterogeneity that have been observed. This finding brings into question the function of the conserved sequences located at the termini of arenavirus genomic RNAs. Our data suggest that, while replication and packaging of the genomic and antigenomic RNA molecules can occur with terminally deleted molecules, mature transcripts may be derived only from full-length templates containing the conserved terminal sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auperin D. D., Compans R. W., Bishop D. H. Nucleotide sequence conservation at the 3' termini of the virion RNA species of New World and Old World arenaviruses. Virology. 1982 Aug;121(1):200–203. doi: 10.1016/0042-6822(82)90130-1. [DOI] [PubMed] [Google Scholar]
- Auperin D. D., McCormick J. B. Nucleotide sequence of the Lassa virus (Josiah strain) S genome RNA and amino acid sequence comparison of the N and GPC proteins to other arenaviruses. Virology. 1989 Feb;168(2):421–425. doi: 10.1016/0042-6822(89)90287-0. [DOI] [PubMed] [Google Scholar]
- Auperin D. D., Romanowski V., Galinski M., Bishop D. H. Sequencing studies of pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J Virol. 1984 Dec;52(3):897–904. doi: 10.1128/jvi.52.3.897-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auperin D., Dimock K., Cash P., Rawls W. E., Leung W. C., Bishop D. H. Analyses of the genomes of prototype pichinde arenavirus and a virulent derivative of Pichinde Munchique: evidence for sequence conservation at the 3' termini of their viral RNA species. Virology. 1982 Jan 15;116(1):363–367. doi: 10.1016/0042-6822(82)90429-9. [DOI] [PubMed] [Google Scholar]
- Barrett A. D., Dimmock N. J. Defective interfering viruses and infections of animals. Curr Top Microbiol Immunol. 1986;128:55–84. doi: 10.1007/978-3-642-71272-2_2. [DOI] [PubMed] [Google Scholar]
- Clegg J. C., Wilson S. M., Oram J. D. Nucleotide sequence of the S RNA of Lassa virus (Nigerian strain) and comparative analysis of arenavirus gene products. Virus Res. 1991 Mar;18(2-3):151–164. doi: 10.1016/0168-1702(91)90015-n. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Mink M. A., Stec D. S. Rescue of synthetic analogs of respiratory syncytial virus genomic RNA and effect of truncations and mutations on the expression of a foreign reporter gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9663–9667. doi: 10.1073/pnas.88.21.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S. J., Southern P. J. Deleted viral RNAs and lymphocytic choriomeningitis virus persistence in vitro. J Gen Virol. 1988 Aug;69(Pt 8):1893–1902. doi: 10.1099/0022-1317-69-8-1893. [DOI] [PubMed] [Google Scholar]
- Franze-Fernández M. T., Zetina C., Iapalucci S., Lucero M. A., Bouissou C., López R., Rey O., Daheli M., Cohen G. N., Zakin M. M. Molecular structure and early events in the replication of Tacaribe arenavirus S RNA. Virus Res. 1987 Jun;7(4):309–324. doi: 10.1016/0168-1702(87)90045-1. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace F. V., Southern P. J. Temporal analysis of transcription and replication during acute infection with lymphocytic choriomeningitis virus. Virology. 1988 Jan;162(1):260–263. doi: 10.1016/0042-6822(88)90419-9. [DOI] [PubMed] [Google Scholar]
- Garcin D., Kolakofsky D. A novel mechanism for the initiation of Tacaribe arenavirus genome replication. J Virol. 1990 Dec;64(12):6196–6203. doi: 10.1128/jvi.64.12.6196-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D., Kolakofsky D. Tacaribe arenavirus RNA synthesis in vitro is primer dependent and suggests an unusual model for the initiation of genome replication. J Virol. 1992 Mar;66(3):1370–1376. doi: 10.1128/jvi.66.3.1370-1376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli P. D., Rivera-Pomar R. V., Lozano M. E., Grau O., Romanowski V. Molecular organization of Junin virus S RNA: complete nucleotide sequence, relationship with other members of the Arenaviridae and unusual secondary structures. J Gen Virol. 1991 Sep;72(Pt 9):2129–2141. doi: 10.1099/0022-1317-72-9-2129. [DOI] [PubMed] [Google Scholar]
- Griffiths C. M., Wilson S. M., Clegg J. C. Sequence of the nucleocapsid protein gene of Machupo virus: close relationship with another South American pathogenic arenavirus, Junín. Arch Virol. 1992;124(3-4):371–377. doi: 10.1007/BF01309817. [DOI] [PubMed] [Google Scholar]
- Han J. H., Shyamala V., Richman K. H., Brauer M. J., Irvine B., Urdea M. S., Tekamp-Olson P., Kuo G., Choo Q. L., Houghton M. Characterization of the terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5' untranslated region and poly(A) tails at the 3' end. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. A., Richards O. C., Summers D. F., Ehrenfeld E. The 5'-terminal nucleotides of hepatitis A virus RNA, but not poliovirus RNA, are required for infectivity. J Virol. 1991 May;65(5):2757–2760. doi: 10.1128/jvi.65.5.2757-2760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iapalucci S., Lopez R., Rey O., Lopez N., Franze-Fernandez M. T., Cohen G. N., Lucero M., Ochoa A., Zakin M. M. Tacaribe virus L gene encodes a protein of 2210 amino acid residues. Virology. 1989 May;170(1):40–47. doi: 10.1016/0042-6822(89)90349-8. [DOI] [PubMed] [Google Scholar]
- Iapalucci S., López N., Rey O., Zakin M. M., Cohen G. N., Franze-Fernández M. T. The 5' region of Tacaribe virus L RNA encodes a protein with a potential metal binding domain. Virology. 1989 Nov;173(1):357–361. doi: 10.1016/0042-6822(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R. J., Hong Z., Strauss J. H. Mutagenesis of the 3' nontranslated region of Sindbis virus RNA. J Virol. 1990 Apr;64(4):1465–1476. doi: 10.1128/jvi.64.4.1465-1476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Rieser L., Vournakis J. N. Labeling of eukaryotic messenger RNA 5' terminus with phosphorus -32: use of tobacco acid pyrophosphatase for removal of cap structures. Gene Amplif Anal. 1981;2:229–251. [PubMed] [Google Scholar]
- Meyer B. J., Southern P. J. Concurrent sequence analysis of 5' and 3' RNA termini by intramolecular circularization reveals 5' nontemplated bases and 3' terminal heterogeneity for lymphocytic choriomeningitis virus mRNAs. J Virol. 1993 May;67(5):2621–2627. doi: 10.1128/jvi.67.5.2621-2627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu M., Schaefer H., Lehmann-Grube F. Homologous interference of lymphocytic choriomeningitis virus: detection and measurement of interference focus-forming units. J Virol. 1976 Oct;20(1):1–8. doi: 10.1128/jvi.20.1.1-8.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Raju L., Hacker D., Garcin D., Compans R., Kolakofsky D. Nontemplated bases at the 5' ends of Tacaribe virus mRNAs. Virology. 1990 Jan;174(1):53–59. doi: 10.1016/0042-6822(90)90053-t. [DOI] [PubMed] [Google Scholar]
- Romanowski V., Matsuura Y., Bishop D. H. Complete sequence of the S RNA of lymphocytic choriomeningitis virus (WE strain) compared to that of Pichinde arenavirus. Virus Res. 1985 Sep;3(2):101–114. doi: 10.1016/0168-1702(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Salvato M. S., Shimomaye E. M. The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology. 1989 Nov;173(1):1–10. doi: 10.1016/0042-6822(89)90216-x. [DOI] [PubMed] [Google Scholar]
- Salvato M., Shimomaye E., Oldstone M. B. The primary structure of the lymphocytic choriomeningitis virus L gene encodes a putative RNA polymerase. Virology. 1989 Apr;169(2):377–384. doi: 10.1016/0042-6822(89)90163-3. [DOI] [PubMed] [Google Scholar]
- Salvato M., Shimomaye E., Southern P., Oldstone M. B. Virus-lymphocyte interactions. IV. Molecular characterization of LCMV Armstrong (CTL+) small genomic segment and that of its variant, Clone 13 (CTL-). Virology. 1988 Jun;164(2):517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- Tse W. T., Forget B. G. Reverse transcription and direct amplification of cellular RNA transcripts by Taq polymerase. Gene. 1990 Apr 16;88(2):293–296. doi: 10.1016/0378-1119(90)90047-u. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Lampert P. W., Oldstone M. B. Prevention of virus-induced cerebellar diseases by defective-interfering lymphocytic choriomeningitis virus. J Infect Dis. 1977 Sep;136(3):391–399. doi: 10.1093/infdis/136.3.391. [DOI] [PubMed] [Google Scholar]
- Widmer G. RNA circularization reveals terminal sequence heterogeneity in a double-stranded RNA virus. Virology. 1993 Mar;193(1):11–15. doi: 10.1006/viro.1993.1098. [DOI] [PubMed] [Google Scholar]
- Wilson S. M., Clegg J. C. Sequence analysis of the S RNA of the African arenavirus Mopeia: an unusual secondary structure feature in the intergenic region. Virology. 1991 Feb;180(2):543–552. doi: 10.1016/0042-6822(91)90068-m. [DOI] [PubMed] [Google Scholar]