Abstract
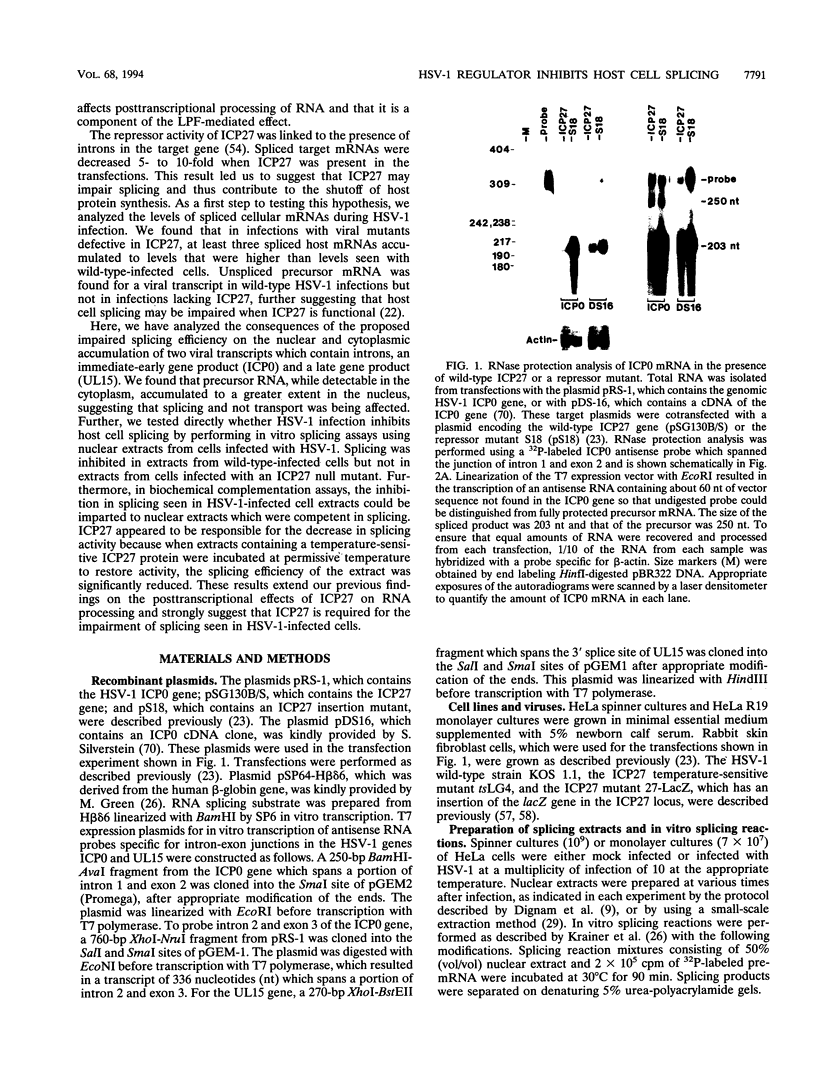
While the majority of metazoan genes and those of the DNA viruses which infect them contain introns which require RNA splicing, herpes simplex virus type 1 (HSV-1) encodes relatively few spliced products. We previously showed that the HSV-1 immediate-early protein ICP27 decreased the levels of spliced target mRNAs in transfections and spliced cellular mRNAs during infection, suggesting that ICP27 may function in impairing host cell splicing. Here, we show that during infections with the wild type, but not in infections with an ICP27 viral mutant termed 27-LacZ, precursor RNA accumulated for a virus transcript which contained introns. Pre-mRNA accumulation in the nucleus was greater than that in the cytoplasm, indicating that splicing rather than transport was affected. Furthermore, splicing of a beta-globin pre-mRNA substrate was inhibited in nuclear extracts from wild-type-infected cells but not in extracts from cells infected with 27-LacZ. The inhibitory activity in extracts from wild-type-infected cells was able to reduce the splicing efficiency of competent extracts in biochemical complementation assays. ICP27 appeared to be responsible for this decrease, because the splicing activity of an extract from cells infected with an ICP27 ts mutant was significantly reduced after incubation of the extract at the permissive temperature to allow renaturation of the conformationally defective ICP27 protein. These results strongly suggest that HSV-1 infection inhibits host cell splicing through the action of ICP27.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso-Caplen F. V., Nemeroff M. E., Qiu Y., Krug R. M. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev. 1992 Feb;6(2):255–267. doi: 10.1101/gad.6.2.255. [DOI] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The cDNA of UL15, a highly conserved herpes simplex virus 1 gene, effectively replaces the two exons of the wild-type virus. J Virol. 1992 Sep;66(9):5621–5626. doi: 10.1128/jvi.66.9.5621-5626.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Reed R. Correspondence between a mammalian spliceosome component and an essential yeast splicing factor. Science. 1993 Oct 1;262(5130):105–108. doi: 10.1126/science.8211113. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Draper K. G., Kelly T. J., Wagner E. K. An unusual spliced herpes simplex virus type 1 transcript with sequence homology to Epstein-Barr virus DNA. J Virol. 1985 May;54(2):317–328. doi: 10.1128/jvi.54.2.317-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Mechanism of action of regulatory proteins encoded by complex retroviruses. Microbiol Rev. 1992 Sep;56(3):375–394. doi: 10.1128/mr.56.3.375-394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin K. D., Knipe D. M. Altered properties of the herpes simplex virus ICP8 DNA-binding protein in cells infected with ICP27 mutant viruses. Virology. 1993 Sep;196(1):1–14. doi: 10.1006/viro.1993.1449. [DOI] [PubMed] [Google Scholar]
- Decker C. J., Parker R. Mechanisms of mRNA degradation in eukaryotes. Trends Biochem Sci. 1994 Aug;19(8):336–340. doi: 10.1016/0968-0004(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. A detailed mutational analysis of Vmw110, a trans-acting transcriptional activator encoded by herpes simplex virus type 1. EMBO J. 1987 Jul;6(7):2069–2076. doi: 10.1002/j.1460-2075.1987.tb02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J Mol Biol. 1988 Jul 5;202(1):87–96. doi: 10.1016/0022-2836(88)90521-9. [DOI] [PubMed] [Google Scholar]
- Everett R. D., Cross A., Orr A. A truncated form of herpes simplex virus type 1 immediate-early protein Vmw110 is expressed in a cell type dependent manner. Virology. 1993 Dec;197(2):751–756. doi: 10.1006/viro.1993.1651. [DOI] [PubMed] [Google Scholar]
- Everett R. D., Dunlop M. Trans activation of plasmid-borne promoters by adenovirus and several herpes group viruses. Nucleic Acids Res. 1984 Aug 10;12(15):5969–5978. doi: 10.1093/nar/12.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986 Nov;67(Pt 11):2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Clark J. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J Gen Virol. 1982 Jul;61(Pt 50):121–125. doi: 10.1099/0022-1317-61-1-121. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., McMenamin M. M. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J Gen Virol. 1984 Jul;65(Pt 7):1225–1228. doi: 10.1099/0022-1317-65-7-1225. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978 Oct;41(1):37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- Fortes P., Beloso A., Ortín J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 1994 Feb 1;13(3):704–712. doi: 10.1002/j.1460-2075.1994.tb06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink R. J., Eisenberg R., Cohen G., Wagner E. K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983 Feb;45(2):634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Hardwicke M. A., Sandri-Goldin R. M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994 Aug;68(8):4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwicke M. A., Vaughan P. J., Sekulovich R. E., O'Conner R., Sandri-Goldin R. M. The regions important for the activator and repressor functions of herpes simplex virus type 1 alpha protein ICP27 map to the C-terminal half of the molecule. J Virol. 1989 Nov;63(11):4590–4602. doi: 10.1128/jvi.63.11.4590-4602.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M., Sinden R. R., Sadler J. R. Herpes simplex virus types 1 and 2 induce shutoff of host protein synthesis by different mechanisms in Friend erythroleukemia cells. J Virol. 1983 Jan;45(1):241–250. doi: 10.1128/jvi.45.1.241-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A. D., Kruper J. A., Frenkel N. Herpes simplex virus virion host shutoff function. J Virol. 1988 Mar;62(3):912–921. doi: 10.1128/jvi.62.3.912-921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Bindereif A., Green M. R. A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal Tech. 1988 Mar-Apr;5(2):22–31. doi: 10.1016/0735-0651(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Legrain P., Chapon C. Interaction between PRP11 and SPP91 yeast splicing factors and characterization of a PRP9-PRP11-SPP91 complex. Science. 1993 Oct 1;262(5130):108–110. doi: 10.1126/science.8211114. [DOI] [PubMed] [Google Scholar]
- Lu Y., Qian X. Y., Krug R. M. The influenza virus NS1 protein: a novel inhibitor of pre-mRNA splicing. Genes Dev. 1994 Aug 1;8(15):1817–1828. doi: 10.1101/gad.8.15.1817. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. A binding consensus: RNA-protein interactions in splicing, snRNPs, and sex. Cell. 1989 Apr 7;57(1):1–3. doi: 10.1016/0092-8674(89)90164-5. [DOI] [PubMed] [Google Scholar]
- Mavromara-Nazos P., Ackermann M., Roizman B. Construction and properties of a viable herpes simplex virus 1 recombinant lacking coding sequences of the alpha 47 gene. J Virol. 1986 Nov;60(2):807–812. doi: 10.1128/jvi.60.2.807-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A. M., McMahan L., Schaffer P. A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989 Jan;63(1):18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J., Phelan A., Loney C., Sandri-Goldin R. M., Clements J. B. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3' processing. J Virol. 1992 Dec;66(12):6939–6945. doi: 10.1128/jvi.66.12.6939-6945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J., Simpson S., Clements J. B. Herpes simplex virus induces a processing factor that stimulates poly(A) site usage. Cell. 1989 Dec 22;59(6):1093–1105. doi: 10.1016/0092-8674(89)90765-4. [DOI] [PubMed] [Google Scholar]
- McMahan L., Schaffer P. A. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J Virol. 1990 Jul;64(7):3471–3485. doi: 10.1128/jvi.64.7.3471-3485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Requirement of protein synthesis for the degradation of host mRNA in Friend erythroleukemia cells infected wtih herpes simplex virus type 1. J Virol. 1978 Sep;27(3):619–627. doi: 10.1128/jvi.27.3.619-627.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Comparison of upstream sequence requirements for positive and negative regulation of a herpes simplex virus immediate-early gene by three virus-encoded trans-acting factors. J Virol. 1987 Jan;61(1):190–199. doi: 10.1128/jvi.61.1.190-199.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985 Mar;53(3):751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroskar A. A., Read G. S. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J Virol. 1987 Feb;61(2):604–606. doi: 10.1128/jvi.61.2.604-606.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. J., Rixon F. J., Everett R. D., Frame M. C., McGeoch D. J. Characterization of the IE110 gene of herpes simplex virus type 1. J Gen Virol. 1986 Nov;67(Pt 11):2365–2380. doi: 10.1099/0022-1317-67-11-2365. [DOI] [PubMed] [Google Scholar]
- Poon A. P., Roizman B. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J Virol. 1993 Aug;67(8):4497–4503. doi: 10.1128/jvi.67.8.4497-4503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981 Jul;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- Qian X. Y., Alonso-Caplen F., Krug R. M. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J Virol. 1994 Apr;68(4):2433–2441. doi: 10.1128/jvi.68.4.2433-2441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Krug R. M. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A). J Virol. 1994 Apr;68(4):2425–2432. doi: 10.1128/jvi.68.4.2425-2432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read G. S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983 May;46(2):498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J Virol. 1988 Oct;62(10):3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990 Apr;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Su L. S., Knipe D. M. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. J Virol. 1989 Aug;63(8):3399–3407. doi: 10.1128/jvi.63.8.3399-3407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Schaffer P. A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987 Mar;61(3):829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Mendoza G. E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992 May;6(5):848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- Schröder H. C., Falke D., Weise K., Bachmann M., Carmo-Fonseca M., Zaubitzer T., Müller W. E. Change of processing and nucleocytoplasmic transport of mRNA in HSV-1-infected cells. Virus Res. 1989 May;13(1):61–78. doi: 10.1016/0168-1702(89)90087-7. [DOI] [PubMed] [Google Scholar]
- Sekulovich R. E., Leary K., Sandri-Goldin R. M. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988 Dec;62(12):4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. L., Hardwicke M. A., Sandri-Goldin R. M. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992 Jan;186(1):74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Smith I. L., Sekulovich R. E., Hardwicke M. A., Sandri-Goldin R. M. Mutations in the activation region of herpes simplex virus regulatory protein ICP27 can be trans dominant. J Virol. 1991 Jul;65(7):3656–3666. doi: 10.1128/jvi.65.7.3656-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Stow E. C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986 Dec;67(Pt 12):2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- Strom T., Frenkel N. Effects of herpes simplex virus on mRNA stability. J Virol. 1987 Jul;61(7):2198–2207. doi: 10.1128/jvi.61.7.2198-2207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Knipe D. M. Herpes simplex virus alpha protein ICP27 can inhibit or augment viral gene transactivation. Virology. 1989 Jun;170(2):496–504. doi: 10.1016/0042-6822(89)90441-8. [DOI] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Vaughan P. J., Thibault K. J., Hardwicke M. A., Sandri-Goldin R. M. The herpes simplex virus immediate early protein ICP27 encodes a potential metal binding domain and binds zinc in vitro. Virology. 1992 Jul;189(1):377–384. doi: 10.1016/0042-6822(92)90720-a. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Flanagan W. M., Devi-Rao G., Zhang Y. F., Hill J. M., Anderson K. P., Stevens J. G. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol. 1988 Dec;62(12):4577–4585. doi: 10.1128/jvi.62.12.4577-4585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Sullivan M., Vande Woude G. F. Structures of two spliced herpes simplex virus type 1 immediate-early mRNA's which map at the junctions of the unique and reiterated regions of the virus DNA S component. J Virol. 1981 Jan;37(1):431–444. doi: 10.1128/jvi.37.1.431-444.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Kenny J. J., Wigdahl B. Antiviral properties of a dominant negative mutant of the herpes simplex virus type 1 regulatory protein ICP0. J Gen Virol. 1992 Nov;73(Pt 11):2955–2961. doi: 10.1099/0022-1317-73-11-2955. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Wigdahl B. Identification of dominant-negative mutants of the herpes simplex virus type 1 immediate-early protein ICP0. J Virol. 1992 Apr;66(4):2261–2267. doi: 10.1128/jvi.66.4.2261-2267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. X., Chen J. X., Silverstein S. Isolation and characterization of a functional cDNA encoding ICP0 from herpes simplex virus type 1. J Virol. 1991 Feb;65(2):957–960. doi: 10.1128/jvi.65.2.957-960.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]











