Abstract
Severe jaundice leading to kernicterus or death in the newborn is the most devastating consequence of glucose-6-phosphate dehydrogenase (EC 1.1.1.49; G-6-PD) deficiency. We asked whether the TA repeat promoter polymorphism in the gene for uridinediphosphoglucuronate glucuronosyltransferase 1 (EC 2.4.1.17; UDPGT1), associated with benign jaundice in adults (Gilbert syndrome), increases the incidence of neonatal hyperbilirubinemia in G-6-PD deficiency. DNA from term neonates was analyzed for UDPGT1 polymorphism (normal homozygotes, heterozygotes, variant homozygotes), and for G-6-PD Mediterranean deficiency. The variant UDPGT1 promoter allele frequency was similar in G-6-PD-deficient and normal neonates. Thirty (22.9%) G-6-PD deficient neonates developed serum total bilirubin ≥ 257 μmol/liter, vs. 22 (9.2%) normals (P = 0.0005). Of those with the normal homozygous UDPGT1 genotype, the incidence of hyperbilirubinemia was similar in G-6-PD-deficients and controls (9.7% and 9.9%). In contrast, in the G-6-PD-deficient neonates, those with the heterozygous or homozygous variant UDPGT1 genotype had a higher incidence of hyperbilirubinemia than corresponding controls (heterozygotes: 31.6% vs. 6.7%, P < 0.0001; variant homozygotes: 50% vs. 14.7%, P = 0.02). Among G-6-PD-deficient infants the incidence of hyperbilirubinemia was greater in those with the heterozygous (31.6%, P = 0.006) or variant homozygous (50%, P = 0.003) UDPGT1 genotype than in normal homozygotes. In contrast, among those normal for G-6-PD, the UDPGT1 polymorphism had no significant effect (heterozygotes: 6.7%; variant homozygotes: 14.7%). Thus, neither G-6-PD deficiency nor the variant UDPGT1 promoter, alone, increased the incidence of hyperbilirubinemia, but both in combination did. This gene interaction may serve as a paradigm of the interaction of benign genetic polymorphisms in the causation of disease.
Keywords: neonatal jaundice, hemolysis, bilirubin conjugation, UDP-glucuronosyltransferase 1, gene interaction
Glucose-6-phosphate dehydrogenase (EC 1.1.1.49; G-6-PD) deficiency is a commonly occurring enzymatic condition estimated to affect hundreds of millions of people worldwide (1). In Israel, G-6-PD deficiency is frequently seen in subsets of Sephardic Jews whose families immigrated from Asia Minor (2, 3). The G-6-PD variant seen in this population group is the Mediterranean type (4).
The most devastating clinical consequence of G-6-PD deficiency is neonatal hyperbilirubinemia which can be severe and result in kernicterus or even death (5–7). The pathogenesis of the jaundice has not been definitively elucidated and is controversial. Some (1) believe that decreased hepatic bilirubin elimination is a key factor, whereas others (8) maintain that increased hemolysis causes the hyperbilirubinemia and that there is no need to invoke liver involvement over and above the normal immaturity of neonatal bilirubin conjugating mechanism. In some cases the hyperbilirubinemia is clearly the result of a hemolytic crisis in response to an identifiable trigger (2, 9, 10). However, the jaundice continues to occur even when contact with all known inciting agents is scrupulously avoided (11). Studies of bilirubin production, reflected by blood carboxyhemoglobin levels, have shown a significantly higher rate of hemolysis in Sephardic Jewish G-6-PD-deficient neonates than in controls (12). Surprisingly, carboxyhemoglobin values were increased to a similar extent in those neonates who developed hyperbilirubinemia and in those who did not. Thus, although the bilirubin load in G-6-PD-deficient neonates is increased, hyperbilirubinemia develops in only a fraction, and the presence or absence of jaundice is not related to the severity of hemolysis. Furthermore, these neonates usually do not develop frank anemia (13, 14) even in the presence of severe hyperbilirubinemia. For these reasons, decreased bilirubin elimination has been suspected to be a key factor in the pathogenesis of the jaundice (1, 15).
In support of this concept, studies of conjugated bilirubin fractions, reflecting intrahepatocytic bilirubin conjugation, have shown a decreased diconjugated bilirubin fraction in hyperbilirubinemic G-6-PD-deficient neonates, compared with hyperbilirubinemic controls (16). This serum conjugated bilirubin fraction profile was reminiscent of that seen in adults with Gilbert syndrome (17), a benign condition of decreased bilirubin conjugation due to diminished activity of the conjugating enzyme uridinediphosphoglucuronate glucuronosyltransferase 1 (EC 2.4.1.17; UDPGT1). Thus we suspected that G-6-PD-deficient neonates who develop hyperbilirubinemia may be those carrying the gene for Gilbert syndrome.
A variant promoter for the gene encoding bilirubin UDPGT1 has recently been described in patients with Gilbert syndrome (18, 19). This promoter contains a two-base-pair addition (TA) in the TATAA element of the promoter, giving rise to seven rather than the more usual six repeats. The extra nucleotides have been shown to decrease the expression of the UDPGT1 gene (18), and the decreased bilirubin conjugation in Gilbert syndrome is the result of this reduced enzyme expression. The aim of the present study was to determine whether the presence of this promoter variant is a risk factor in the pathogenesis of G-6-PD-deficiency-associated neonatal hyperbilirubinemia. We hypothesized that the combined effect of an increased bilirubin load, due to excessive hemolysis in G-6-PD deficiency, and decreased bilirubin conjugation, due to reduced expression of UDPGT1, would increase the incidence of hyperbilirubinemia in G-6-PD-deficient neonates in an allele dose-dependent fashion.
METHODS
Patient Population and Clinical Protocol.
A cohort of healthy term neonates, both males and females, at high risk for G-6-PD deficiency by virtue of their Sephardic Jewish maternal lineage, formed the patient group for this study. Neonates with any medical condition other than G-6-PD deficiency known to exacerbate the incidence or severity of neonatal hyperbilirubinemia, such as direct Coombs positive hemolytic anemia, maternal diabetes, cephalhematoma, or sepsis, were excluded from the study. Immediately following delivery of the placenta, umbilical cord blood was collected into an EDTA-containing tube, and DNA was extracted from the cells. The neonates were observed clinically, and with serum total bilirubin determinations as necessary, both as inpatients and subsequently as outpatients, until stabilization of serum total bilirubin values. Phototherapy was commenced if serum total bilirubin values exceeded 257 μmol/liter, and exchange transfusion was performed if the bilirubin values remained >342 μmol/liter for more than several hours, despite phototherapy. The study protocol was approved by the Shaare Zedek Medical Center’s Institutional Review Board.
Definitions.
The G-6-PD-deficient group was composed of male neonates hemizygous for the Mediterranean type of G-6-PD deficiency (G-6-PD Mediterranean563T) and female neonates either hetero- or homozygous for this type of G-6-PD deficiency. The control G-6-PD normal group consisted of neonates from the same population group, who had been entered into the study and found not to have this mutation. For the purpose of the study, hyperbilirubinemia was defined as a serum total bilirubin ≥257 μmol/liter, while neonates whose serum total bilirubin remained ≤255 μmol/liter or who were not sufficiently jaundiced so as to warrant a serum bilirubin determination, were classified nonhyperbilirubinemic. Peak serum total bilirubin values are not reported, because phototherapy in the hyperbilirubinemic patients most likely prevented the levels from reaching their natural peak. Patients were classified according to the gene encoding the UDPGT1 promoter as normal homozygotes (6/6), bearing the sequence (TA)6TAA, variant homozygotes (7/7), with the sequence (TA)7TAA, and heterozygotes (6/7), with one of each allele.
Data Analysis.
The variant UDPGT1 allele frequency was determined by calculating the percentage of variant promoters of the total number of UDPGT1 alleles in the population studied. Relative risk was calculated to estimate the risk of developing hyperbilirubinemia in any study subgroup relative to that of the appropriate control subgroup. A 95% confidence interval (CI), with jaundice as dependent variable, and UDPGT1 genotype and G-6-PD status as independent variables, was used as a measure of the statistical precision of each relative risk. Significance was achieved when the 95% CIs were wholly above or below 1. If the 95% CI straddled 1, the results were regarded as not significant (NS). Logistic regression analysis was performed to determine whether birth weight, gestational age, or sex were confounding variables for the development of hyperbilirubinemia. Categorical variables were compared using χ2 analysis or Fisher’s exact test, as appropriate, while continuous variables were analyzed using Student’s t test, significance being defined as P < 0.05.
Laboratory Methods.
All laboratory tests were performed at the Shaare Zedek Medical Center, except for molecular analysis of the G-6-PD gene, which was carried out at the Scripps Research Institute. Serum total bilirubin values were determined by reflectance spectrophotometry using a Kodak Ektachem analyzer with Clinical Chemistry Slides (Ektachem 750 XRC Analyzer, Eastman Kodak, Rochester, NY). Blood groups and direct Coombs testing were performed by routine laboratory techniques. DNA was extracted from umbilical cord blood by using a high-salt extraction procedure (20).
Molecular Classification of the G-6-PD Status.
A 127-bp fragment of DNA containing nucleotide 563, the nucleotide mutated in G-6-PD Mediterranean (21), was PCR amplified (22) using oligonucleotides 7 and 8 (23) as primers. The presence or absence of the 563T mutation was determined by allele-specific oligonucleotide hybridization. Each amplified sample was spotted onto an 8 × 12 grid on each of two wetted nitrocellulose membranes along with control samples with and without the 563T mutation. For the purpose of monitoring the washing of the samples (see below), separate small filters were spotted with positive and negative controls. The papers were soaked for 2 min in 1.5 M NaCl/0.5 M NaOH, 5 min in 1.5 M NaCl/0.5 M Tris⋅HCl, pH 8.0, and 30 s in 0.2 M Tris⋅HCl, pH 7.5/2× SSC (1× SSC = 0.15 M NaCl/0.015 M sodium citrate, pH 7). The membranes were then either dried in a vacuum oven or fixed with a UV Stratalinker (Stratagene). After prehybridization in 6× SSC/5× Denhardt’s solution/0.02 M sodium phosphate buffer, pH 7.0, containing 0.5 mg/ml salmon sperm DNA and 0.1% SDS, they were hybridized at 42°C overnight in the same solution without Denhardt’s solution but containing approximately 107 cpm of the following 32P-labeled oligonucleotides: 5′-CATCTCCTCCCTGTTCCGTGA-3′ (the normal sequence), or 5′-CATCTTCTCCCTGTTCCGTG-3′ (the mutant sequence, mutation underlined).
The calculated melting temperature of the normal sequence is 66°C and that of the mutant sequence is 62°C. The membranes were washed in 6× SSC/0.1% SDS beginning at 10°C below their calculated melting temperature, raising the temperature and monitoring with a Geiger counter until the negative controls became unreactive. The membranes were then dried and placed on film.
Molecular Classification of Gilbert Syndrome Status.
Identification of individuals with the variant promoter for the gene bilirubin UDPGT1 was performed by amplification of the promoter region for the gene using PCR, with primers Bili x, 5′-ATTAACTTGGTGTCGATTGG-3′, and Bili z, 5′-AGCCATGGCGGCCTTTGCTC-3′, which amplify a 90-bp (wild-type) or 92-bp (variant) DNA fragment. PCR mixtures were prepared on ice and were immediately denatured at 94°C (1 min), annealed at 54°C (1 min), and extended at 72°C (45 s) for 2 cycles, followed by 33 cycles of 95°C, 54°C, 72°C (30 s each) and a final incubation at 72°C for 10 min, using the thermostable DNA polymerase Super-Therm (LPI, London, U.K.) in a reaction buffer containing 1.5 mM MgCl2, supplied by the manufacturer. The reaction products were separated by electrophoresis on a 10% polyacrylamide gel and stained with ethidium bromide.
RESULTS
The study took place between January and December, 1996. During this period 189 male and 182 female newborns were entered into the study. Of these, 59 males were found to be G-6-PD-deficient hemizygotes, 53 females G-6-PD-deficient heterozygotes, and 19 females G-6-PD-deficient homozygotes. Thus the entire cohort was composed of 131 G-6-PD-deficient neonates and 240 control G-6-PD-normal neonates. Although the incidence of hyperbilirubinemia was slightly lower in the G-6-PD Mediterranean female heterozygotes than in the deficient homozygotes or male hemizygotes, this value was not significantly lower, and the data from subjects with all of these genotypes were pooled. Neither sex nor birth weight was associated with an increased risk of hyperbilirubinemia. Although increasing gestational age was associated with a decreasing incidence of hyperbilirubinemia, this effect was similar in G-6-PD-deficient and normal groups, and it was therefore not a confounder in the relationships among neonatal hyperbilirubinemia, G-6-PD status, and UDPGT1 variant promoter status. The results are summarized in Table 1.
Table 1.
Incidence of hyperbilirubinemia as a function of presence of the G-6-PD Mediterranean mutation and the UDPGT1 genotype
| G-6-PD Mediterranean | Incidence of UDPGT1 genotype
|
|||
|---|---|---|---|---|
| 6/6 | 6/7 | 7/7 | Total | |
| Present (deficient) | 6/62 (9.7%) | 18/57 (31.6%) | 6/12 (50%) | 30/131 (22.9%) |
| Absent (normal) | 10/101 (9.9%) | 7/105 (6.7%) | 5/34 (14.7%) | 22/240 (9.2%) |
| Significance | NS | P < 0.0001 | P = 0.02 | P = 0.0005 |
6/6, Homozygous normal UDPGT1 genotype; 6/7, heterozygous variant UDPGT1 genotype; 7/7, homozygous UDPGT1 genotype.
Thirty (22.7%) of the G-6-PD-deficient neonates developed hyperbilirubinemia, vs. 22 (9.2%) of the controls (P = 0.0005). The relative risk for developing hyperbilirubinemia was significantly higher for the entire G-6-PD-deficient group, compared with the G-6-PD-normal group (2.5 with a 95% CI of 1.5–4.1). The variant UDPGT1 promoter allele frequency was similar in the G-6-PD-deficient and G-6-PD-normal groups (0.317 and 0.363, respectively, NS) and in males and females (0.323 and 0.338 respectively, NS).
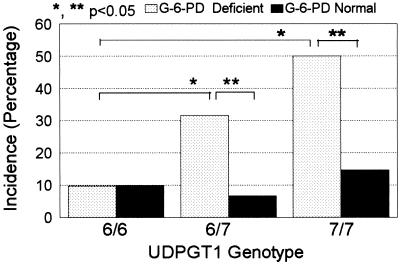
The incidence of hyperbilirubinemia in the G-6-PD-deficient and normal control groups was compared in each of the three UDPGT1 genotypes (Fig. 1, Table 1). In the G-6-PD-deficient and control patients who were homozygous normal for the UDPGT1 gene, the incidences were almost identical. However, among those heterozygous or homozygous for the variant UDPGT1 promoter, the incidence of hyperbilirubinemia was significantly higher in the G-6-PD-deficient neonates than in the corresponding G-6-PD-normal controls. Accordingly, among the G-6-PD-deficient infants the incidence of hyperbilirubinemia was significantly higher in the UDPGT1 variant heterozygotes and homozygotes (P = 0.006 and P = 0.003, respectively) compared with those with the normal homozygous genotype. In contrast were the infants with normal G-6-PD, in whom, although the incidence of hyperbilirubinemia was slightly higher in those with the homozygous variant genotype, a variant UDPGT1 promoter did not have a statistically significant effect.
Figure 1.
Incidence (percentage) of hyperbilirubinemia (serum total bilirubin ≥257 μmol/liter) in G-6-PD-deficient neonates and normal controls, stratified for the three genotypes of the UDGPT1 variant promoter.
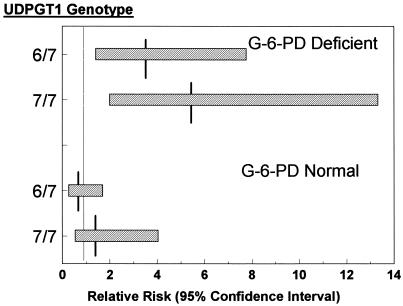
Using neonates homozygous normal for UDPGT1 as the baseline, it can be seen from Fig. 2 that the relative risk for developing hyperbilirubinemia for G-6-PD-deficient neonates was increased when they were both either heterozygous or homozygous for the variant promoter (relative risk = 3.3 with a 95% CI of 1.4–7.8, and relative risk = 5.2 with a 95% CI of 2.0–13.3, respectively). However, this was not the case in infants without G-6-PD mutations (relative risk = 0.7 with a 95% CI of 0.3–1.7, and relative risk = 1.5 with a 95% CI of 0.5–4.0), for those heterozygous and homozygous for the variant UDPGT1 promoter, respectively). The effect of the variant UDGPT1 gene on the incidence of neonatal hyperbilirubinemia was greater in the male hemizygotes than in the female heterozygotes. However, the small number of female G-6-PD-deficient homozygotes with any variant UDPGT1 promoter precluded definitive analysis.
Figure 2.
Added risk of hyperbilirubinemia attributable to the variant UDGPT1 allele in the G-6-PD-deficient and normal control groups. UDPGT1 hetero- and variant homozygotes were compared with normal homozygotes (defined as relative risk 1). The figure illustrates the relative risks and their 95% CI (x-axis) for each UDPGT1 genotype (y-axis). Horizontal bars, 95% CIs; vertical lines, relative risk.
DISCUSSION
Serum total bilirubin values at any time are dependent on bilirubin production, on the one hand, and bilirubin elimination, on the other. As we have pointed out, hemolysis cannot in and of itself be the only factor contributing to the pathogenesis of jaundice in newborn G-6-PD-deficient infants. We therefore suspected that decreased bilirubin conjugation may play a major role in its development. Bilirubin is eliminated from the body by conjugation with glucuronic acid in the liver by the enzyme UDPGT1. The monoconjugated and diconjugated bilirubin can then be excreted into the bile. The gene coding for UDPGT1 has been located at the telomeric end (q37) of human chromosome 2 and its molecular organization has recently been described (24).
Gilbert syndrome is a mild and common form of UDPGT1 deficiency (25). From 6% to 10% of the general population are clinically affected and have serum total bilirubin levels that may fluctuate up to 50 μmol/liter, and even higher during intercurrent illness. No other abnormalities of liver function are encountered. Hepatic bilirubin glucuronosyltransferase activity is decreased in affected persons by 60–70% (26). Serum bilirubin fractions show a greater than normal unconjugated bilirubin fraction (>90%) (27) and a decreased diconjugated bilirubin component (17).
Recently, Bosma et al. (18) and subsequently Monaghan et al. (19) documented a polymorphism in the promoter region of the gene for UDPGT1 that appears to be present in most patients with Gilbert syndrome. Subjects with Gilbert syndrome are homozygous for a variant TATAA element that contains two extra nucleotides, TA, in the upstream promoter region of the gene. Additional persons who were homozygous for the TATAA variant did not have the clinical picture of Gilbert syndrome, but did have significantly higher serum bilirubin values than normals or heterozygotes, indicating that the variant TATAA may be associated with those individuals on the high end of the normal bilirubin distribution range.
As expression of UDPGT1 is frequently reduced in newborns, it seems logical to suspect an association between the variant TATAA box of the gene for UDPGT1 and the development of neonatal hyperbilirubinemia. Bancroft et al. (28), using a transcutaneous jaundice index, found that homozygotes for the variant promoter had a greater increase of the index over the first few days of life than did heterozygotes or normals homozygotes. However, peak bilirubin levels were not significantly increased among the homozygotes. In our study, both the incidence of and the relative risk for developing hyperbilirubinemia in the G-6-PD-deficient neonates increased in an allele dose-dependent fashion, with the addition of one or two variant UDPGT1 promoter alleles. In contrast, the incidence of hyperbilirubinemia in the infants without G-6-PD deficiency was only minimally affected, if at all, by the presence or absence of such alleles. Furthermore, G-6-PD-deficient neonates without the variant UDPGT1 promoter were at no higher risk for neonatal hyperbilirubinemia than the population without G-6-PD deficiency, with or without a variant promoter. We have thus shown that presence of a variant UDPGT1 promoter is actively involved in the pathogenesis of G-6-PD-deficiency-associated neonatal hyperbilirubinemia, and that neither G-6-PD deficiency nor presence of the variant allele alone was sufficient to increase the risk of hyperbilirubinemia.
The reason for this increased effect of the variant UDPGT1 allele in the G-6-PD-deficient state is not yet fully known. It is possible that the liver of a neonate hetero- or homozygous for the variant promoter may be able to handle a normal bilirubin load, but not the larger load imposed by increased hemolysis in G-6-PD deficiency. However, in a previous study (12) carboxyhemoglobin values (measuring the rate of bilirubin production as a reflection of red cell destruction), and peak serum total bilirubin values were positively correlated in infants without G-6-PD deficiency, implying a major contributory role of hemolysis to the development of jaundice in that group. In contrast, the rate of red cell destruction and jaundice did not correlate in the G-6-PD-deficient neonates, indicating that factors other than the rate of hemolysis are important in determining which infants develop jaundice. The additive effect of presence of a variant UDPGT1 promoter allele is apparently one such factor. It is feasible that G-6-PD deficiency exerts its effect in alternate ways, possibly by affecting the rate of hepatic bilirubin elimination. Indeed, Chan et al. (29), Brunetti et al. (30), and Oluboyede et al. (31) have shown that liver parenchymal activity of the G-6-PD enzyme is low in G-6-PD-deficient individuals.
The clinical manifestations of the polymorphic forms of G-6-PD deficiency are chiefly acute hemolytic anemia, on the one hand, and neonatal jaundice, on the other. However, G-6-PD deficiency results in acute hemolysis only when an external stress such as drug administration or infection is superimposed upon the underlying genetic abnormality. Thus, G-6-PD deficiency has long been regarded as a paradigm for the interaction between genes and the environment (32). Now we find that G-6-PD deficiency alone does not produce neonatal jaundice, but that here, too, an additional factor is required, a mutation in UDPGT1. Alone, this mutation is quite benign, causing the mild jaundice of Gilbert syndrome, but together with G-6-PD deficiency, it can result neonatal hyperbilirubinemia severe enough to cause kernicterus. While such an association does not necessarily imply causation, the facts that the effect of the variant UDPGT1 allele was dose dependent and that the trend to develop hyperbilirubinemia was more pronounced in the G-6-PD-deficient hemizygotes and homozygotes than in the female heterozygotes support the concept that the association is biologically relevant. Thus far, few such examples of gene–gene interactions have been described. It was suggested (33) that the bimodal hemolytic response of G-6-PD-deficient patients to thiazolesulfone (34) might be due to the N-acetylation polymorphism, and interactions between different mutations involving globin chain synthesis are well known and relatively easily understood (35).
Multigenic diseases represent a great frontier of clinical genetics. The interaction of the genes for G-6-PD deficiency and for Gilbert syndrome may serve as a simplified model of such diseases because a major proportion, although presumably not all, of the effect is exerted by only two mutations. Such simplified models may further our understanding of this very difficult area.
Acknowledgments
We thank the delivery room nursing staff of the Shaare Zedek Medical Center for their assistance in collecting the umbilical cord blood samples, Dr. Glenn Gourley for his assistance while we were establishing the PCR-based assay for the UDPGT1 promoter mutation, and Dr. Nina Lonshakova and Mrs. Beryl Westwood for technical assistance. The study was supported in part by a grant from the General Research Fund, Shaare Zedek Medical Center, and by National Institutes of Health Grants RR00833 and HL25552.
ABBREVIATIONS
- G-6-PD
glucose-6-phosphate dehydrogenase
- UDPGT1
uridinediphosphoglucuronate glucuronosyltransferase 1
- 6/6
UDPGT1 normal homozygotes
- 6/7
heterozygotes for variant UDPGT1 promoter
- 7/7
homozygotes for variant UDPGT1 promoter
- CI
confidence interval
- NS
not significant
References
- 1.Beutler E. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- 2.Sheba C, Szeinberg A, Ramot B, Adam A, Ashkenazi I. Am J Public Health. 1962;52:1101–1105. doi: 10.2105/ajph.52.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan M, Hammerman C, Rudensky B, Kvit R, Abramov A. Arch Dis Child. 1994;71:F59–F60. doi: 10.1136/fn.71.1.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oppenheim A, Jury C L, Rund D, Vulliamy T J, Luzzatto L. Hum Genet. 1993;91:293–294. doi: 10.1007/BF00218277. [DOI] [PubMed] [Google Scholar]
- 5.Doxiadis S A, Fessas P, Valaes T, Mastrokalos N. Lancet. 1961;i:297–301. doi: 10.1016/s0140-6736(61)91476-3. [DOI] [PubMed] [Google Scholar]
- 6.Slusher T M, Vreman H J, McLaren D W, Lewison L J, Brown A K, Stevenson D K. J Pediatr. 1995;126:102–108. doi: 10.1016/s0022-3476(95)70510-4. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald M G. Pediatrics. 1995;96:734–738. [PubMed] [Google Scholar]
- 8.Valaes T. Acta Paediatr Suppl. 1994;394:58–76. doi: 10.1111/j.1651-2227.1994.tb13216.x. [DOI] [PubMed] [Google Scholar]
- 9.Freier S, Mayer K, Levene C, Abramov A. Arch Dis Child. 1965;40:280–283. doi: 10.1136/adc.40.211.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinkham W H, Oski F A. Pediatrics. 1996;97:707–710. [PubMed] [Google Scholar]
- 11.Kaplan M, Abramov A. Pediatrics. 1992;90:401–405. [PubMed] [Google Scholar]
- 12.Kaplan M, Vreman H J, Hammerman C, Leiter C, Abramov A, Stevenson D K. Br J Haematol. 1996;93:822–827. doi: 10.1046/j.1365-2141.1996.d01-1745.x. [DOI] [PubMed] [Google Scholar]
- 13.Valaes T, Karaklis A, Stravrakakis D, Bavela-Stravrakakis K, Perakis A, Doxiadis S A. Pediatr Res. 1969;3:448–458. doi: 10.1203/00006450-196909000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Meloni T, Costa S, Cutillo S. Acta Haematol. 1975;54:284–288. doi: 10.1159/000208087. [DOI] [PubMed] [Google Scholar]
- 15.Panizon F. Lancet. 1960;ii:1093. [Google Scholar]
- 16.Kaplan M, Rubaltelli F F, Hammerman C, Vilei M T, Leiter C, Abramov A, Muraca M. J Pediatr. 1996;128:695–697. doi: 10.1016/s0022-3476(96)80138-7. [DOI] [PubMed] [Google Scholar]
- 17.Muraca M, Fevery J, Blankaert N. Gastroenterology. 1987;92:309–317. doi: 10.1016/0016-5085(87)90123-5. [DOI] [PubMed] [Google Scholar]
- 18.Bosma P J, Chowdhury J R, Bakker C, Gantla S, de Boer A, Oostra B A, Lindhout D, Tytgat G N J, Jansen P L M, Oude Elferink R J P, Chowdhury N R. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 19.Monaghan G, Ryan M, Seddon R, Hume R, Burchell B. Lancet. 1996;347:578–581. doi: 10.1016/s0140-6736(96)91273-8. [DOI] [PubMed] [Google Scholar]
- 20.Miller S A, Dykes D D, Polesky H F. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vulliamy T J, D’Urso M, Battistuzzi G, Estrada M, Foulkes N, Martini G, Calabro V, Poggi V, Giordano R, Town M, Luzzatto L, Persico M G. Proc Natl Acad Sci USA. 1988;85:5171–5175. doi: 10.1073/pnas.85.14.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vives-Corrons J-L, Kuhl W, Pujades M A, Beutler E. Am J Hum Genet. 1990;47:575–579. [PMC free article] [PubMed] [Google Scholar]
- 23.Hirono A, Beutler E. J Clin Invest. 1989;83:343–346. doi: 10.1172/JCI113881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritter J K, Chen F, Sheen Y Y, Tran H M, Kimura M T, Owens I S. J Biol Chem. 1992;267:3257–3261. [PubMed] [Google Scholar]
- 25.Chowdhury J R, Chowdhury N R. Gastroenterology. 1993;105:288–293. doi: 10.1016/0016-5085(93)90041-a. [DOI] [PubMed] [Google Scholar]
- 26.Black M, Billing B H. N Engl J Med. 1969;280:1266–1271. doi: 10.1056/NEJM196906052802303. [DOI] [PubMed] [Google Scholar]
- 27.Sieg A, Stiehl A, Raedsch R, Ullrich D, Messmer B, Kommerell B. Clin Chim Acta. 1986;154:41–48. doi: 10.1016/0009-8981(86)90086-0. [DOI] [PubMed] [Google Scholar]
- 28.Bancroft, J. D., Kreamer, B. & Gourley, G. R. (1997) J. Pediatr., in press. [DOI] [PubMed]
- 29.Chan T K, Todd D, Wong C, C. J Lab Clin Med. 1965;66:937–941. [PubMed] [Google Scholar]
- 30.Brunetti P, Rossetti R, Broccia G. Clin Ther. 1960;32:338–350. [PubMed] [Google Scholar]
- 31.Oluboyede O A, Esan G J F, Francis T I, Luzzatto L. J Lab Clin Med. 1979;93:783–789. [PubMed] [Google Scholar]
- 32.Motulsky A G. J Am Med Assoc. 1957;165:835–837. doi: 10.1001/jama.1957.72980250010016. [DOI] [PubMed] [Google Scholar]
- 33.Magon A M, Leipzig R M, Zannoni V G, Brewer G J. J Lab Clin Med. 1981;97:764–770. [PubMed] [Google Scholar]
- 34.Dern R J, Beutler E, Alving A S. J Lab Clin Med. 1955;45:30–39. [PubMed] [Google Scholar]
- 35.Beutler E. In: Hematology. Beutler E, Lichtman M A, Coller B S, Kipps T J, editors. New York: McGraw–Hill; 1995. pp. 616–654. [Google Scholar]