Abstract
Oral hairy leukoplakia (HLP) lesions frequently contain defective Epstein-Barr virus (EBV) genomes with deletions in the EBNA-2 gene that abundantly replicate and persist within the lesion. To characterize these viral strains and recombinant variants, the EBNA-2 gene in EBV DNA from several different HLP biopsy specimens was analyzed. Amplification of EBNA-2 coding sequences by PCR demonstrated the presence in HLP of intact EBNA-2 genes as well as a variety of internally deleted variants of both EBNA-2A and EBNA-2B. Some of the deletion variants evolved within the HLP lesion from intact EBNA-2 genes, while other variants appeared to be transmissible strains that directly infected the lesion. Intrastrain recombination within the HLP lesion also generated variation within the EBNA-2 polyproline region. Cloning and sequencing of HLP cDNA demonstrated transcription from the internally deleted EBNA-2 open reading frame, indicating that these variant genes are expressed in HLP. Comparative analysis of the HLP EBNA-2 sequences confirmed previous findings of EBV coinfection with multiple types and strains. Sequence variation of these wild-type genes demonstrated that EBNA-2A sequences distinguish at least two separate strains and a variety of substrains of EBV type 1. Two of the HLP EBNA-2A sequences contained amino acid changes in a cytotoxic T-cell epitope within an otherwise highly conserved region of the gene. These data indicate that EBV coinfection, strain variation, and recombination within the EBNA-2 gene are common features of HLP and suggest that the expression of internally deleted EBNA-2 variants could contribute to EBV pathogenesis in permissive infection.
Full text
PDF

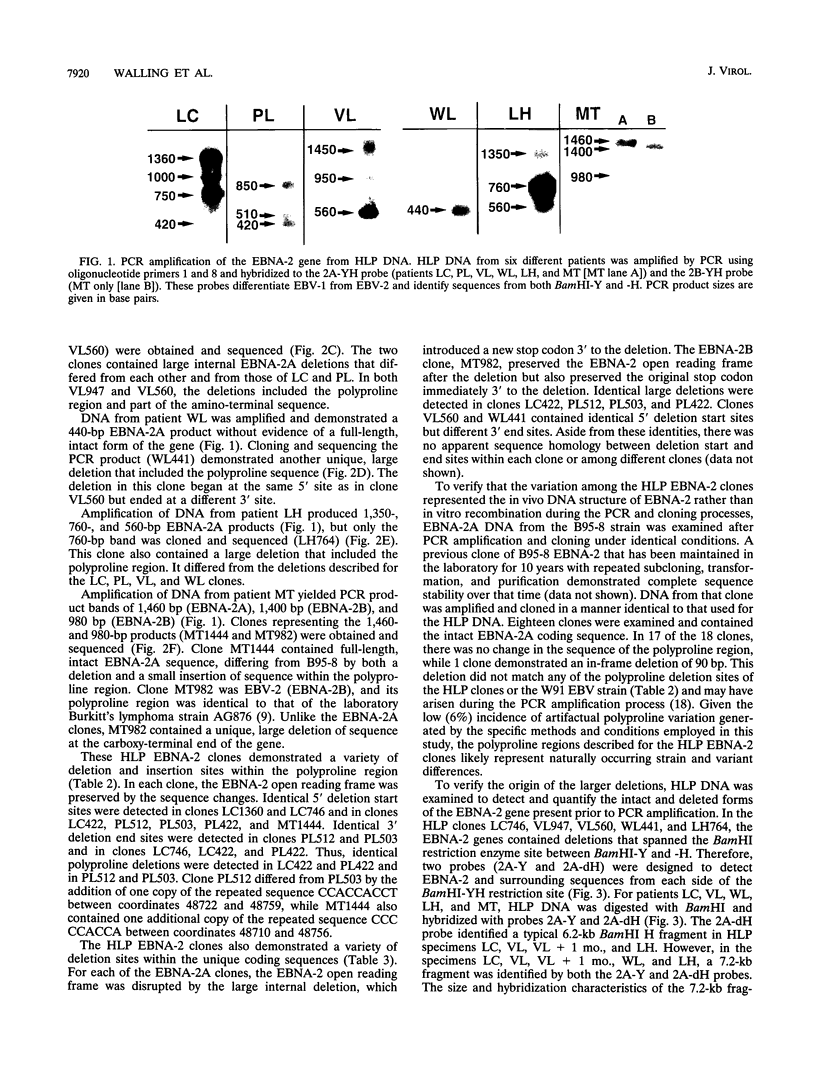
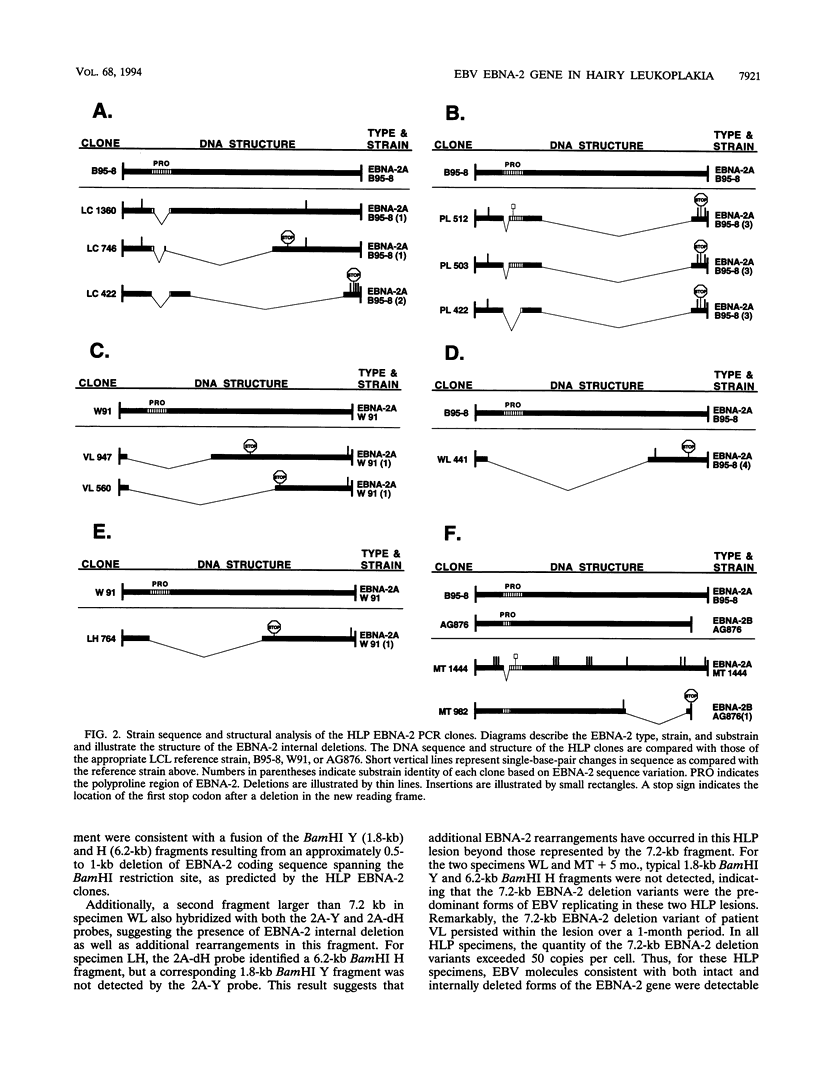


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken C., Sengupta S. K., Aedes C., Moss D. J., Sculley T. B. Heterogeneity within the Epstein-Barr virus nuclear antigen 2 gene in different strains of Epstein-Barr virus. J Gen Virol. 1994 Jan;75(Pt 1):95–100. doi: 10.1099/0022-1317-75-1-95. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Zimber U., Hudewentz J., Epstein M. A. Comparison of Epstein-Barr virus strains of different origin by analysis of the viral DNAs. J Virol. 1980 Sep;35(3):603–618. doi: 10.1128/jvi.35.3.603-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. S., Bornkamm G. W., zur Hausen H. Structure of defective DNA molecules in Epstein-Barr virus preparations from P3HR-1 cells. J Virol. 1984 Jul;51(1):199–207. doi: 10.1128/jvi.51.1.199-207.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Kieff E. An Epstein-Barr virus nuclear protein 2 domain essential for transformation is a direct transcriptional activator. J Virol. 1991 Nov;65(11):5880–5885. doi: 10.1128/jvi.65.11.5880-5885.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Wang F., Mannick J., Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavioli R., Kurilla M. G., de Campos-Lima P. O., Wallace L. E., Dolcetti R., Murray R. J., Rickinson A. B., Masucci M. G. Multiple HLA A11-restricted cytotoxic T-lymphocyte epitopes of different immunogenicities in the Epstein-Barr virus-encoded nuclear antigen 4. J Virol. 1993 Mar;67(3):1572–1578. doi: 10.1128/jvi.67.3.1572-1578.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan K., Rajadurai P., Resnick L., Raab-Traub N. Epstein-Barr virus small nuclear RNAs are not expressed in permissively infected cells in AIDS-associated leukoplakia. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8790–8794. doi: 10.1073/pnas.87.22.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Oosterveer M. A., Klein G., Ernberg I. EBNA size polymorphism can be used to trace Epstein-Barr virus spread within families. J Virol. 1990 Oct;64(10):4703–4708. doi: 10.1128/jvi.64.10.4703-4708.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Oosterveer M. A., Weimar W., Sintnicolaas K., Sizoo W., Bolhuis R. L., Ernberg I. Detection of multiple 'Ebnotypes' in individual Epstein-Barr virus carriers following lymphocyte transformation by virus derived from peripheral blood and oropharynx. J Gen Virol. 1994 Jan;75(Pt 1):85–94. doi: 10.1099/0022-1317-75-1-85. [DOI] [PubMed] [Google Scholar]
- Greenspan J. S., Greenspan D., Lennette E. T., Abrams D. I., Conant M. A., Petersen V., Freese U. K. Replication of Epstein-Barr virus within the epithelial cells of oral "hairy" leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985 Dec 19;313(25):1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- Heller M., Dambaugh T., Kieff E. Epstein-Barr virus DNA. IX. Variation among viral DNAs from producer and nonproducer infected cells. J Virol. 1981 May;38(2):632–648. doi: 10.1128/jvi.38.2.632-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Heller M., van Santen V., Kieff E. Simple repeat array in Epstein-Barr virus DNA encodes part of the Epstein-Barr nuclear antigen. Science. 1983 Jun 24;220(4604):1396–1398. doi: 10.1126/science.6304878. [DOI] [PubMed] [Google Scholar]
- Huang L. M., Jeang K. T. Long-range jumping of incompletely extended polymerase chain fragments generates unexpected products. Biotechniques. 1994 Feb;16(2):242-4, 246. [PubMed] [Google Scholar]
- Hwang C. B., Shillitoe E. J. Analysis of complex mutations induced in cells by herpes simplex virus type-1. Virology. 1991 Apr;181(2):620–629. doi: 10.1016/0042-6822(91)90895-i. [DOI] [PubMed] [Google Scholar]
- Jenson H. B., Farrell P. J., Miller G. Sequences of the Epstein-Barr Virus (EBV) large internal repeat form the center of a 16-kilobase-pair palindrome of EBV (P3HR-1) heterogeneous DNA. J Virol. 1987 May;61(5):1495–1506. doi: 10.1128/jvi.61.5.1495-1506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R., Burrows S. R., Kurilla M. G., Jacob C. A., Misko I. S., Sculley T. B., Kieff E., Moss D. J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992 Jul 1;176(1):169–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau R., Middeldorp J., Farrell P. J. Epstein-Barr virus gene expression in oral hairy leukoplakia. Virology. 1993 Aug;195(2):463–474. doi: 10.1006/viro.1993.1397. [DOI] [PubMed] [Google Scholar]
- Ling P. D., Rawlins D. R., Hayward S. D. The Epstein-Barr virus immortalizing protein EBNA-2 is targeted to DNA by a cellular enhancer-binding protein. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9237–9241. doi: 10.1073/pnas.90.20.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P. D., Ryon J. J., Hayward S. D. EBNA-2 of herpesvirus papio diverges significantly from the type A and type B EBNA-2 proteins of Epstein-Barr virus but retains an efficient transactivation domain with a conserved hydrophobic motif. J Virol. 1993 Jun;67(6):2990–3003. doi: 10.1128/jvi.67.6.2990-3003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Heston L., Countryman J. P3HR-1 Epstein-Barr virus with heterogeneous DNA is an independent replicon maintained by cell-to-cell spread. J Virol. 1985 Apr;54(1):45–52. doi: 10.1128/jvi.54.1.45-52.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. E., Edwards R. H., Walling D. M., Raab-Traub N. Sequence variation in the Epstein-Barr virus latent membrane protein 1. J Gen Virol. 1994 Oct;75(Pt 10):2729–2740. doi: 10.1099/0022-1317-75-10-2729. [DOI] [PubMed] [Google Scholar]
- Patton D. F., Shirley P., Raab-Traub N., Resnick L., Sixbey J. W. Defective viral DNA in Epstein-Barr virus-associated oral hairy leukoplakia. J Virol. 1990 Jan;64(1):397–400. doi: 10.1128/jvi.64.1.397-400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M., Gradoville L., Heston L., Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982 Dec;44(3):834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C., Howe J. G., Speck S. H., Miller G. Influence of Burkitt's lymphoma and primary B cells on latent gene expression by the nonimmortalizing P3J-HR-1 strain of Epstein-Barr virus. J Virol. 1989 Apr;63(4):1531–1539. doi: 10.1128/jvi.63.4.1531-1539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Hummel M., Braun D., Birkenbach M., Kieff E. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Young L., Martin B., Chatman T., Kieff E., Rickinson A., Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990 Sep;64(9):4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvej K., Krenács L., Hamilton-Dutoit S. J., Rindum J. L., Pindborg J. J., Pallesen G. Epstein-Barr virus latent and replicative gene expression in oral hairy leukoplakia. Histopathology. 1992 May;20(5):387–395. doi: 10.1111/j.1365-2559.1992.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Sculley T. B., Apolloni A., Hurren L., Moss D. J., Cooper D. A. Coinfection with A- and B-type Epstein-Barr virus in human immunodeficiency virus-positive subjects. J Infect Dis. 1990 Sep;162(3):643–648. doi: 10.1093/infdis/162.3.642. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Shirley P., Sloas M., Raab-Traub N., Israele V. A transformation-incompetent, nuclear antigen 2-deleted Epstein-Barr virus associated with replicative infection. J Infect Dis. 1991 May;163(5):1008–1015. doi: 10.1093/infdis/163.5.1008. [DOI] [PubMed] [Google Scholar]
- Stephen D., Jones C., Schofield J. P. A rapid method for isolating high quality plasmid DNA suitable for DNA sequencing. Nucleic Acids Res. 1990 Dec 25;18(24):7463–7464. doi: 10.1093/nar/18.24.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Felix D. H., Wray D., Southam J. C., Cubie H. A., Crawford D. H. Epstein-Barr virus gene expression and epithelial cell differentiation in oral hairy leukoplakia. Am J Pathol. 1991 Dec;139(6):1369–1380. [PMC free article] [PubMed] [Google Scholar]
- Tomkinson B., Robertson E., Yalamanchili R., Longnecker R., Kieff E. Epstein-Barr virus recombinants from overlapping cosmid fragments. J Virol. 1993 Dec;67(12):7298–7306. doi: 10.1128/jvi.67.12.7298-7306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling D. M., Edmiston S. N., Sixbey J. W., Abdel-Hamid M., Resnick L., Raab-Traub N. Coinfection with multiple strains of the Epstein-Barr virus in human immunodeficiency virus-associated hairy leukoplakia. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6560–6564. doi: 10.1073/pnas.89.14.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlrab F., Chatterjee S., Wells R. D. The herpes simplex virus 1 segment inversion site is specifically cleaved by a virus-induced nuclear endonuclease. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6432–6436. doi: 10.1073/pnas.88.15.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Dawson C. W., Clark D., Rupani H., Busson P., Tursz T., Johnson A., Rickinson A. B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988 May;69(Pt 5):1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- de Campos-Lima P. O., Gavioli R., Zhang Q. J., Wallace L. E., Dolcetti R., Rowe M., Rickinson A. B., Masucci M. G. HLA-A11 epitope loss isolates of Epstein-Barr virus from a highly A11+ population. Science. 1993 Apr 2;260(5104):98–100. doi: 10.1126/science.7682013. [DOI] [PubMed] [Google Scholar]