Abstract
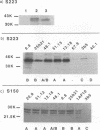
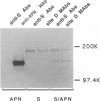
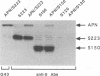
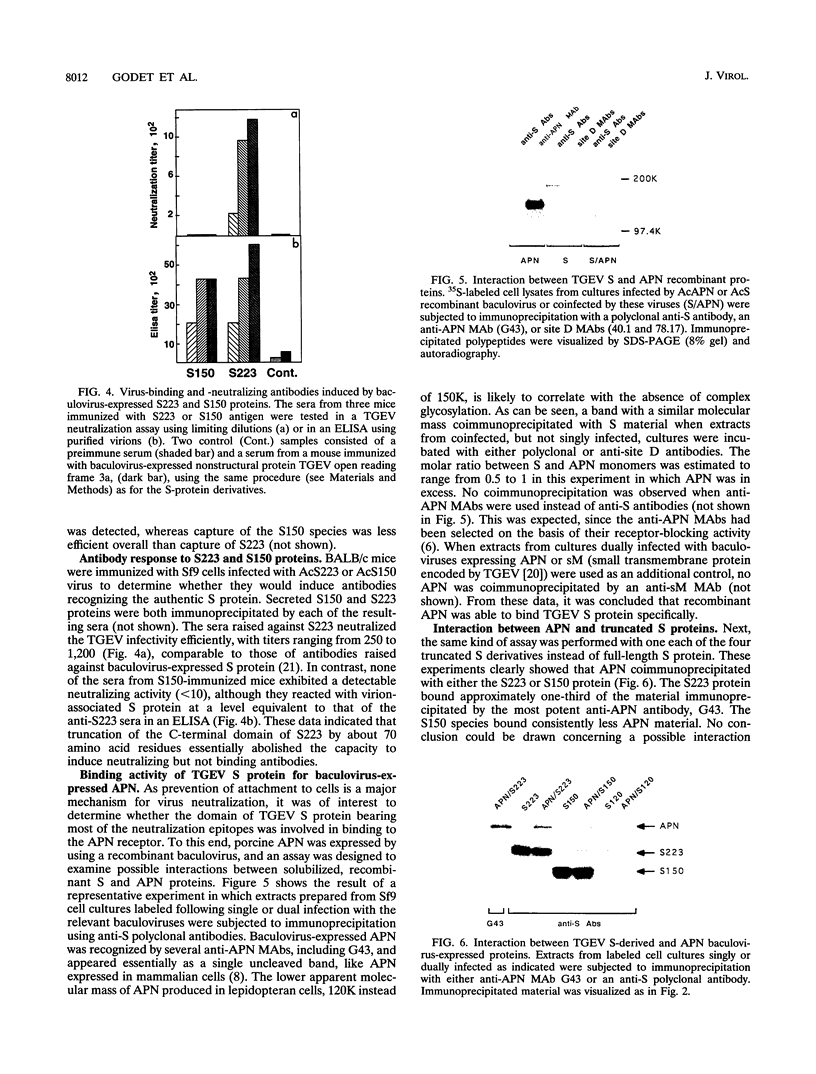
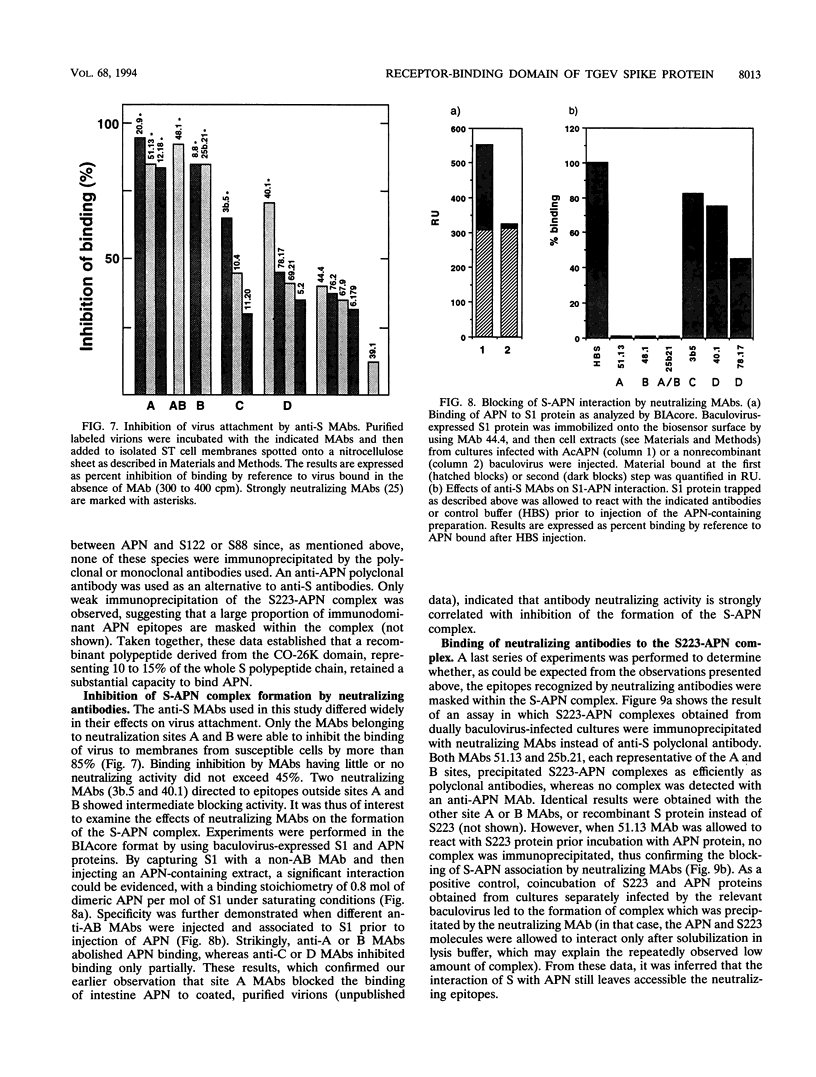
The spike glycoprotein (S) of coronavirus, the major target for virus-neutralizing antibodies, is assumed to mediate the attachment of virions to the host cell. A 26-kilodalton fragment proteolytically cleaved from transmissible gastroenteritis virus (TGEV) S protein was previously shown to bear two adjacent antigenic sites, A and B, both defined by high-titer neutralizing antibodies. Recombinant baculoviruses expressing C-terminal truncations of the 26-kilodalton region were used to localize functionally important determinants in the S protein primary structure. Two overlapping 223- and 150-amino-acid-long products with serine 506 as a common N terminus expressed all of the site A and B epitopes and induced virus-binding antibodies. Coexpression of one of these truncated protein S derivatives with aminopeptidase N (APN), a cell surface molecule acting as a receptor for TGEV, led to the formation of a complex which could be immunoprecipitated by anti-S antibodies. These data provide evidence that major neutralization-mediating and receptor-binding determinants reside together within a domain of the S protein which behaves like an independent module. In spite of their ability to prevent S-APN interaction, the neutralizing antibodies appeared to recognize a preformed complex, thus indicating that antibody- and receptor-binding determinants should be essentially distinct. Together these findings bring new insight into the molecular mechanism of TGEV neutralization.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Correa I., Gebauer F., Bullido M. J., Suñ C., Baay M. F., Zwaagstra K. A., Posthumus W. P., Lenstra J. A., Enjuanes L. Localization of antigenic sites of the E2 glycoprotein of transmissible gastroenteritis coronavirus. J Gen Virol. 1990 Feb;71(Pt 2):271–279. doi: 10.1099/0022-1317-71-2-271. [DOI] [PubMed] [Google Scholar]
- Daniel C., Talbot P. J. Protection from lethal coronavirus infection by affinity-purified spike glycoprotein of murine hepatitis virus, strain A59. Virology. 1990 Jan;174(1):87–94. doi: 10.1016/0042-6822(90)90057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot R. J., Van Leen R. W., Dalderup M. J., Vennema H., Horzinek M. C., Spaan W. J. Stably expressed FIPV peplomer protein induces cell fusion and elicits neutralizing antibodies in mice. Virology. 1989 Aug;171(2):493–502. doi: 10.1016/0042-6822(89)90619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Kut E., Sjöström H., Noren O., Laude H. Determinants essential for the transmissible gastroenteritis virus-receptor interaction reside within a domain of aminopeptidase-N that is distinct from the enzymatic site. J Virol. 1994 Aug;68(8):5216–5224. doi: 10.1128/jvi.68.8.5216-5224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L. K., Sjöström H., Norén O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992 Jun 4;357(6377):417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Laude H. Antigenic structure of transmissible gastroenteritis virus. II. Domains in the peplomer glycoprotein. J Gen Virol. 1986 Jul;67(Pt 7):1405–1418. doi: 10.1099/0022-1317-67-7-1405. [DOI] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Sjöström H., Noren O., Laude H. Further characterization of aminopeptidase-N as a receptor for coronaviruses. Adv Exp Med Biol. 1993;342:293–298. doi: 10.1007/978-1-4615-2996-5_45. [DOI] [PubMed] [Google Scholar]
- Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J Virol. 1990 Nov;64(11):5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Laude H. Carbohydrate-induced conformational changes strongly modulate the antigenicity of coronavirus TGEV glycoproteins S and M. Virus Res. 1991 Jul;20(2):107–120. doi: 10.1016/0168-1702(91)90103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Rasschaert D., Godet M., Gelfi J., Laude H. Four major antigenic sites of the coronavirus transmissible gastroenteritis virus are located on the amino-terminal half of spike glycoprotein S. J Gen Virol. 1990 Jun;71(Pt 6):1313–1323. doi: 10.1099/0022-1317-71-6-1313. [DOI] [PubMed] [Google Scholar]
- Duarte M., Gelfi J., Lambert P., Rasschaert D., Laude H. Genome organization of porcine epidemic diarrhoea virus. Adv Exp Med Biol. 1993;342:55–60. doi: 10.1007/978-1-4615-2996-5_9. [DOI] [PubMed] [Google Scholar]
- Duarte M., Laude H. Sequence of the spike protein of the porcine epidemic diarrhoea virus. J Gen Virol. 1994 May;75(Pt 5):1195–1200. doi: 10.1099/0022-1317-75-5-1195. [DOI] [PubMed] [Google Scholar]
- Dveksler G. S., Pensiero M. N., Dieffenbach C. W., Cardellichio C. B., Basile A. A., Elia P. E., Holmes K. V. Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1716–1720. doi: 10.1073/pnas.90.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley J. K., Parker S. E., Bloom F., Buchmeier M. J. The V5A13.1 envelope glycoprotein deletion mutant of mouse hepatitis virus type-4 is neuroattenuated by its reduced rate of spread in the central nervous system. Virology. 1992 Mar;187(1):178–188. doi: 10.1016/0042-6822(92)90306-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet M., L'Haridon R., Vautherot J. F., Laude H. TGEV corona virus ORF4 encodes a membrane protein that is incorporated into virions. Virology. 1992 Jun;188(2):666–675. doi: 10.1016/0042-6822(92)90521-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet M., Rasschaert D., Laude H. Processing and antigenicity of entire and anchor-free spike glycoprotein S of coronavirus TGEV expressed by recombinant baculovirus. Virology. 1991 Dec;185(2):732–740. doi: 10.1016/0042-6822(91)90544-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G., Correa I., Melgosa M. P., Bullido M. J., Enjuanes L. Critical epitopes in transmissible gastroenteritis virus neutralization. J Virol. 1986 Oct;60(1):131–139. doi: 10.1128/jvi.60.1.131-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts P. A., Ayres M. D., Possee R. D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990 Oct 11;18(19):5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M. Coronavirus: organization, replication and expression of genome. Annu Rev Microbiol. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- Laude H., Chapsal J. M., Gelfi J., Labiau S., Grosclaude J. Antigenic structure of transmissible gastroenteritis virus. I. Properties of monoclonal antibodies directed against virion proteins. J Gen Virol. 1986 Jan;67(Pt 1):119–130. doi: 10.1099/0022-1317-67-1-119. [DOI] [PubMed] [Google Scholar]
- Laude H., Van Reeth K., Pensaert M. Porcine respiratory coronavirus: molecular features and virus-host interactions. Vet Res. 1993;24(2):125–150. [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- McNamee M. G. Isolation and characterization of cell membranes. Biotechniques. 1989 May;7(5):466–475. [PubMed] [Google Scholar]
- Parker S. E., Gallagher T. M., Buchmeier M. J. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology. 1989 Dec;173(2):664–673. doi: 10.1016/0042-6822(89)90579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford D. J., Britton P. Intracellular processing of the porcine coronavirus transmissible gastroenteritis virus spike protein expressed by recombinant vaccinia virus. Virology. 1991 Jun;182(2):765–773. doi: 10.1016/0042-6822(91)90617-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasschaert D., Duarte M., Laude H. Porcine respiratory coronavirus differs from transmissible gastroenteritis virus by a few genomic deletions. J Gen Virol. 1990 Nov;71(Pt 11):2599–2607. doi: 10.1099/0022-1317-71-11-2599. [DOI] [PubMed] [Google Scholar]
- Rasschaert D., Laude H. The predicted primary structure of the peplomer protein E2 of the porcine coronavirus transmissible gastroenteritis virus. J Gen Virol. 1987 Jul;68(Pt 7):1883–1890. doi: 10.1099/0022-1317-68-7-1883. [DOI] [PubMed] [Google Scholar]
- Revision of the taxonomy of the Coronavirus, Torovirus and Arterivirus genera. Arch Virol. 1994;135(1-2):227–237. doi: 10.1007/BF01309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W., Cavanagh D., Horzinek M. C. Coronaviruses: structure and genome expression. J Gen Virol. 1988 Dec;69(Pt 12):2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- Suñ C., Jiménez G., Correa I., Bullido M. J., Gebauer F., Smerdou C., Enjuanes L. Mechanisms of transmissible gastroenteritis coronavirus neutralization. Virology. 1990 Aug;177(2):559–569. doi: 10.1016/0042-6822(90)90521-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez C. M., Gebauer F., Suñ C., Mendez A., Dopazo J., Enjuanes L. Genetic evolution and tropism of transmissible gastroenteritis coronaviruses. Virology. 1992 Sep;190(1):92–105. doi: 10.1016/0042-6822(92)91195-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Heijnen L., Zijderveld A., Horzinek M. C., Spaan W. J. Intracellular transport of recombinant coronavirus spike proteins: implications for virus assembly. J Virol. 1990 Jan;64(1):339–346. doi: 10.1128/jvi.64.1.339-346.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. I., Fleming J. O., Lai M. M. Sequence analysis of the spike protein gene of murine coronavirus variants: study of genetic sites affecting neuropathogenicity. Virology. 1992 Feb;186(2):742–749. doi: 10.1016/0042-6822(92)90041-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismiller D. G., Sturman L. S., Buchmeier M. J., Fleming J. O., Holmes K. V. Monoclonal antibodies to the peplomer glycoprotein of coronavirus mouse hepatitis virus identify two subunits and detect a conformational change in the subunit released under mild alkaline conditions. J Virol. 1990 Jun;64(6):3051–3055. doi: 10.1128/jvi.64.6.3051-3055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager C. L., Ashmun R. A., Williams R. K., Cardellichio C. B., Shapiro L. H., Look A. T., Holmes K. V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992 Jun 4;357(6377):420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., Lai M. M. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J Virol. 1992 Oct;66(10):6194–6199. doi: 10.1128/jvi.66.10.6194-6199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., Luytjes W., Horzinek M. C., van der Zeijst B. A., Spaan W. J., Lenstra J. A. Evidence for a coiled-coil structure in the spike proteins of coronaviruses. J Mol Biol. 1987 Aug 20;196(4):963–966. doi: 10.1016/0022-2836(87)90422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]