Abstract
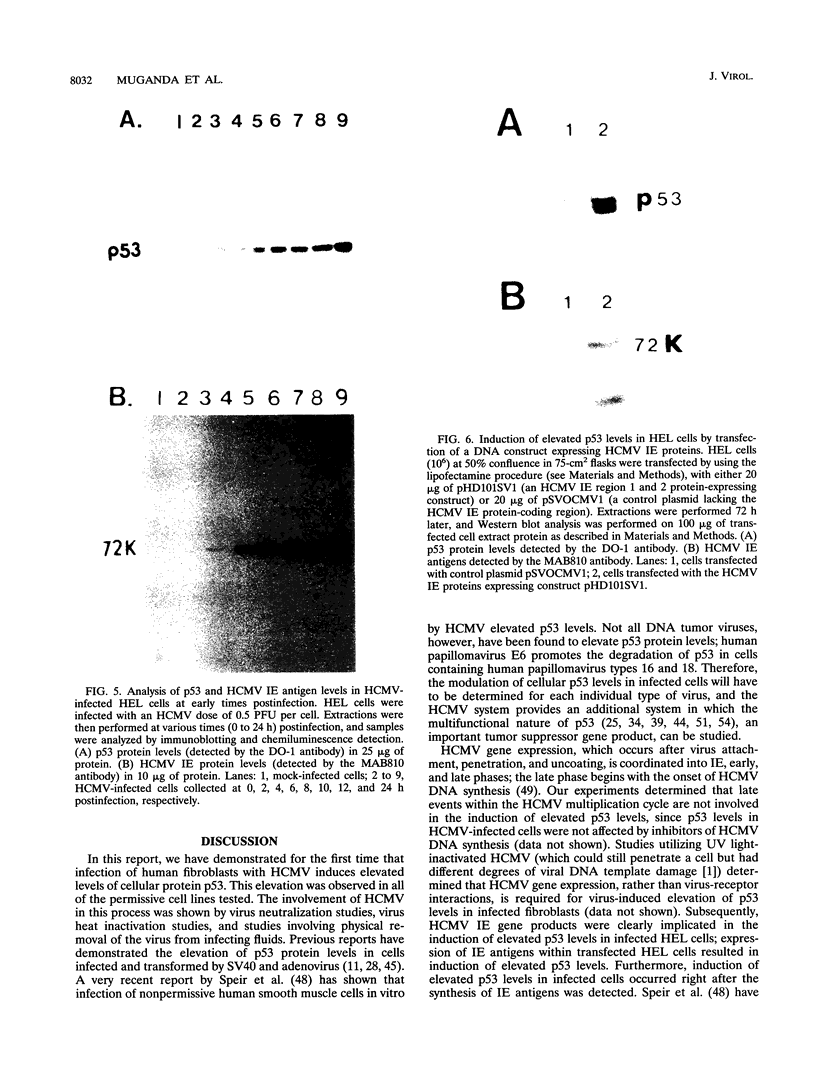
Human cytomegalovirus (HCMV), like other DNA tumor viruses, induces morphological transformation of cells in vitro and stimulates host cell macromolecular synthesis in infected cells. Since other DNA tumor viruses, such as simian virus 40 and adenovirus, have previously been shown to interact with cellular protein p53, we investigated whether infection of cells by HCMV would modulate cellular p53 levels. Our results indicate that HCMV elevates cellular p53 levels on the order of 10- to 20-fold in infected fibroblasts. The induction of elevated p53 levels was dependent upon the presence of active virus and was prevented by neutralizing antibody. The induction of elevated p53 levels was determined not to be due to virus-receptor interactions or HCMV late events. The induction of elevated p53 levels commenced at immediate-early times of the HCMV multiplication cycle (6 h postinfection) and reached maximal levels by 24 h postinfection, before most of the HCMV DNA synthesis was initiated. HCMV immediate-early proteins were clearly shown to be responsible for elevating p53 levels in infected fibroblasts; expression of HCMV immediate-early region 1 and 2 proteins resulted in elevation of p53 levels in transfected human fibroblasts. This is the first report of increased p53 levels caused by HCMV in infected fibroblasts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AbuBakar S., Au W. W., Legator M. S., Albrecht T. Induction of chromosome aberrations and mitotic arrest by cytomegalovirus in human cells. Environ Mol Mutagen. 1988;12(4):409–420. doi: 10.1002/em.2860120409. [DOI] [PubMed] [Google Scholar]
- Albrecht T., Boldogh I., Fons M., Lee C. H., AbuBakar S., Russell J. M., Au W. W. Cell-activation responses to cytomegalovirus infection relationship to the phasing of CMV replication and to the induction of cellular damage. Subcell Biochem. 1989;15:157–202. [PubMed] [Google Scholar]
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- Boldogh I., AbuBakar S., Deng C. Z., Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol. 1991 Mar;65(3):1568–1571. doi: 10.1128/jvi.65.3.1568-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh I., AbuBakar S., Millinoff D., Deng C. Z., Albrecht T. Cellular oncogene activation by human cytomegalovirus. Lack of correlation with virus infectivity and immediate early gene expression. Arch Virol. 1991;118(3-4):163–177. doi: 10.1007/BF01314027. [DOI] [PubMed] [Google Scholar]
- Boldogh I., Gönczöl E., Váczi L. Transformation of hamster embryonic fibroblast cells by UV-irradiated human cytomegalovirus. Acta Microbiol Acad Sci Hung. 1978;25(4):269–275. [PubMed] [Google Scholar]
- Boldogh I., Huang E. S., Baskar J. F., Gergely L., Albrecht T. Oncogenic transformation by cellular DNA isolated from human cytomegalovirus-infected cells. Intervirology. 1992;34(2):62–73. doi: 10.1159/000150264. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Sturzbecher H. W., Addison C., Palmer C., Rudge K., Jenkins J. R. Mouse p53 inhibits SV40 origin-dependent DNA replication. Nature. 1987 Oct 1;329(6138):458–460. doi: 10.1038/329458a0. [DOI] [PubMed] [Google Scholar]
- Braithwaite A., Nelson C., Skulimowski A., McGovern J., Pigott D., Jenkins J. Transactivation of the p53 oncogene by E1a gene products. Virology. 1990 Aug;177(2):595–605. doi: 10.1016/0042-6822(90)90525-v. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Crawford L. The 53,000-dalton cellular protein and its role in transformation. Int Rev Exp Pathol. 1983;25:1–50. [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Kaplan A. S. Physiological state of human embryonic lung cells affects their response to human cytomegalovirus. J Virol. 1977 Jul;23(1):126–132. doi: 10.1128/jvi.23.1.126-132.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Haug M. Evidence for free and metabolically stable p53 protein in nuclear subfractions of simian virus 40-transformed cells. Mol Cell Biol. 1986 Jun;6(6):2233–2240. doi: 10.1128/mcb.6.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Geder K. M., Lausch R., O'Neill F., Rapp F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science. 1976 Jun 11;192(4244):1134–1137. doi: 10.1126/science.179143. [DOI] [PubMed] [Google Scholar]
- Ho M. Epidemiology of cytomegalovirus infections. Rev Infect Dis. 1990 Sep-Oct;12 (Suppl 7):S701–S710. doi: 10.1093/clinids/12.supplement_7.s701. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Mar E. C., Boldogh I., Baskar J. The oncogenicity of human cytomegalovirus. Birth Defects Orig Artic Ser. 1984;20(1):193–211. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990 Aug;177(2):419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Maltzman W., Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984 Sep;4(9):1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- Mazeron M. C., Jahn G., Plachter B. Monoclonal antibody E-13 (M-810) to human cytomegalovirus recognizes an epitope encoded by exon 2 of the major immediate early gene. J Gen Virol. 1992 Oct;73(Pt 10):2699–2703. doi: 10.1099/0022-1317-73-10-2699. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Baserga R. Expression of the p53 protein during the cell cycle of human peripheral blood lymphocytes. Exp Cell Res. 1985 Sep;160(1):31–46. doi: 10.1016/0014-4827(85)90233-2. [DOI] [PubMed] [Google Scholar]
- Mercer W. E. Cell cycle regulation and the p53 tumor suppressor protein. Crit Rev Eukaryot Gene Expr. 1992;2(3):251–263. [PubMed] [Google Scholar]
- Montenarh M. Biochemical, immunological, and functional aspects of the growth-suppressor/oncoprotein p53. Crit Rev Oncog. 1992;3(3):233–256. [PubMed] [Google Scholar]
- Muganda-Ojiaku P. M., Huang E. S. Alteration of protein phosphorylation patterns in cell lines morphologically transformed by human cytomegalovirus. Cancer Biochem Biophys. 1987 May;9(2):179–189. [PubMed] [Google Scholar]
- Rapp F., Robbins D. Cytomegalovirus and human cancer. Birth Defects Orig Artic Ser. 1984;20(1):175–192. [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Oren M., Levine A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Popliker M., Webb C. G., Oren M. p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol. 1985 Oct;5(10):2851–2855. doi: 10.1128/mcb.5.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert J. M., Stillman B. Analysis of a protein-binding domain of p53. Mol Cell Biol. 1993 Jun;13(6):3811–3820. doi: 10.1128/mcb.13.6.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schooley R. T. Cytomegalovirus in the setting of infection with human immunodeficiency virus. Rev Infect Dis. 1990 Sep-Oct;12 (Suppl 7):S811–S819. doi: 10.1093/clinids/12.supplement_7.s811. [DOI] [PubMed] [Google Scholar]
- Speir E., Modali R., Huang E. S., Leon M. B., Shawl F., Finkel T., Epstein S. E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994 Jul 15;265(5170):391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- Su L., Hershberger R. J., Weissman I. L. LYAR, a novel nucleolar protein with zinc finger DNA-binding motifs, is involved in cell growth regulation. Genes Dev. 1993 May;7(5):735–748. doi: 10.1101/gad.7.5.735. [DOI] [PubMed] [Google Scholar]
- Tiemann F., Deppert W. Stabilization of the tumor suppressor p53 during cellular transformation by simian virus 40: influence of viral and cellular factors and biological consequences. J Virol. 1994 May;68(5):2869–2878. doi: 10.1128/jvi.68.5.2869-2878.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S. J., Anderson C. W., Mercer W. E., Appella E. The p53 tumor suppressor protein, a modulator of cell proliferation. J Biol Chem. 1992 Aug 5;267(22):15259–15262. [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N Engl J Med. 1971 Jul 29;285(5):267–274. doi: 10.1056/NEJM197107292850507. [DOI] [PubMed] [Google Scholar]
- Wilcock D., Lane D. P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991 Jan 31;349(6308):429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Zhan Q., Carrier F., Fornace A. J., Jr Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol Cell Biol. 1993 Jul;13(7):4242–4250. doi: 10.1128/mcb.13.7.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Gutsch D., Kenney S. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol Cell Biol. 1994 Mar;14(3):1929–1938. doi: 10.1128/mcb.14.3.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]