Abstract
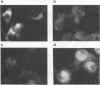
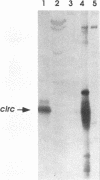
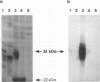
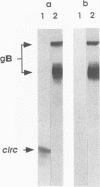
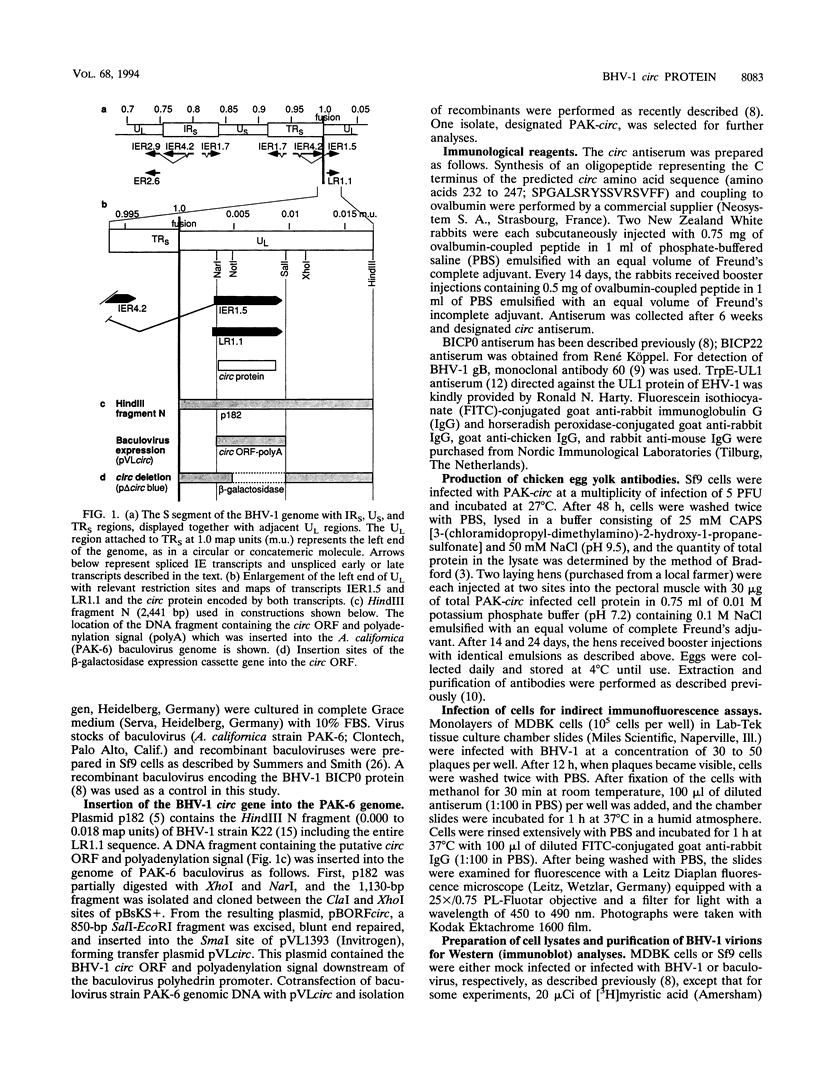
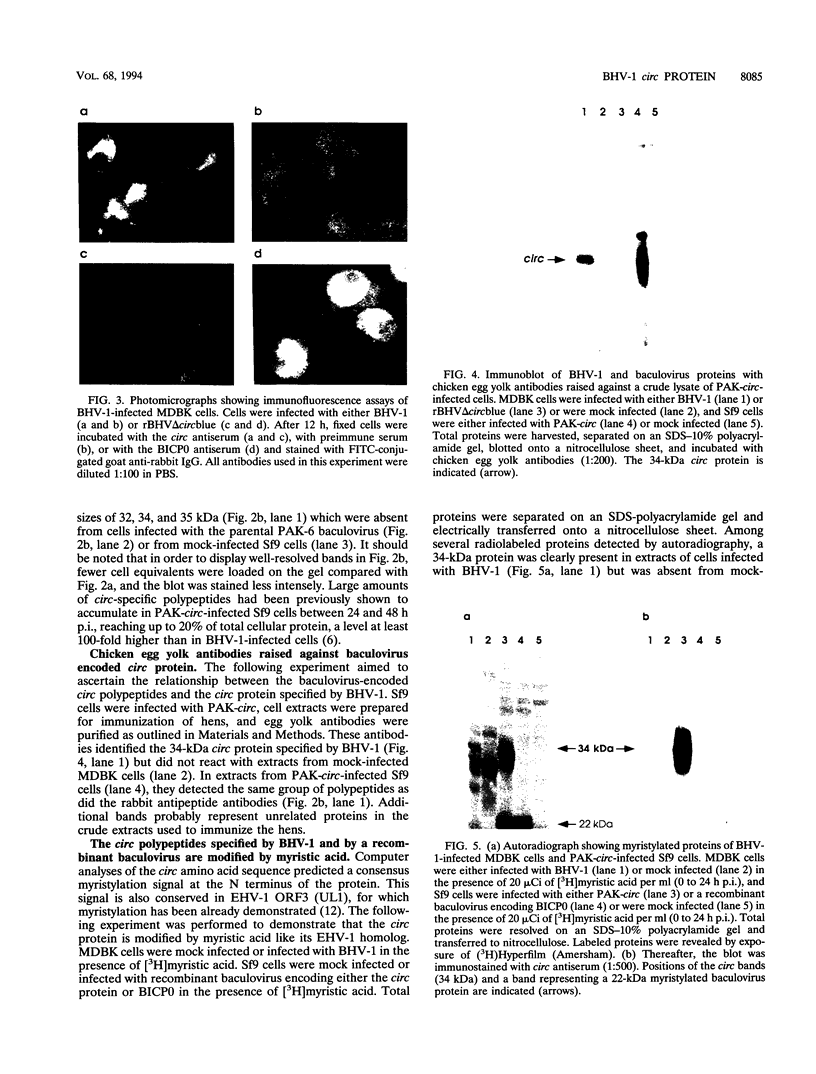
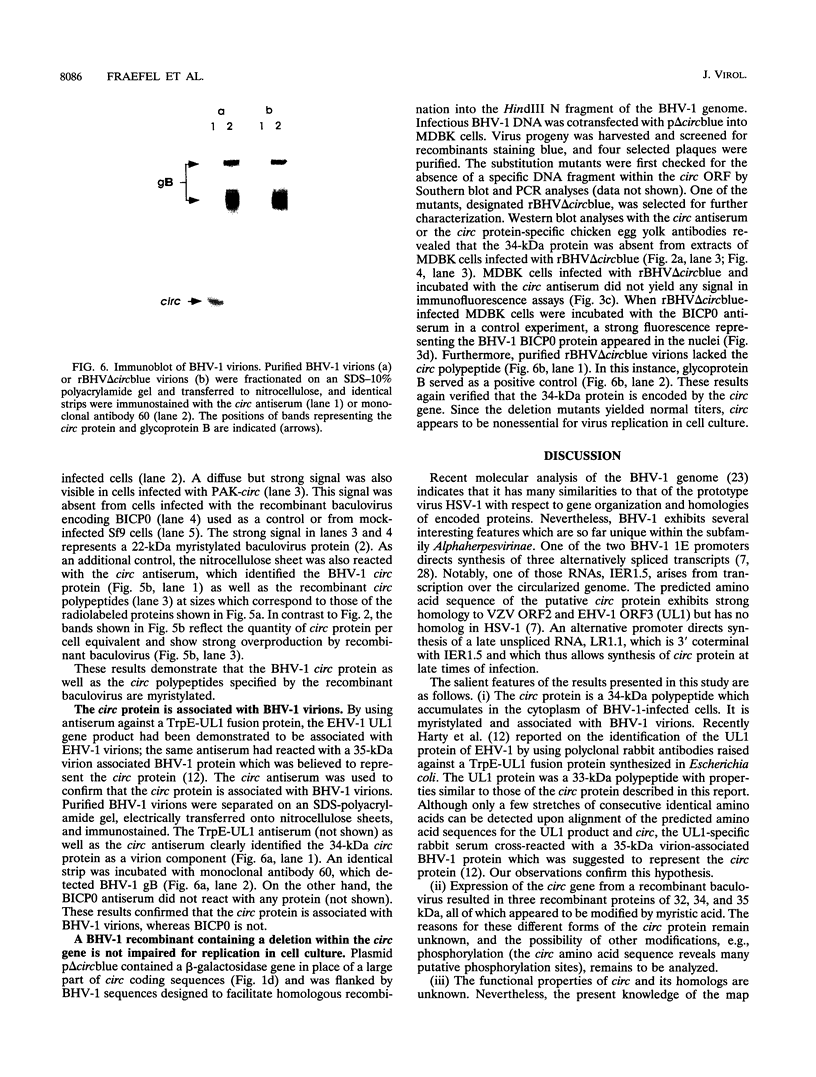
We have recently reported immediate-early (IE) transcription over covalently joined genome ends of bovine herpesvirus 1 (BHV-1). A spliced 1.5-kb IE RNA (IER1.5) is coterminal with an unspliced 1.1-kb late RNA (LR1.1) which is transcribed from the left end of the genome. Sequence analysis reveals an open reading frame common to IER1.5 and LR1.1 predicted to encode the 247-amino-acid circ polypeptide. This paper reports on the identification of circ as a protein. Using a rabbit antiserum raised against a synthetic oligopeptide representing the carboxy terminus of the predicted circ polypeptide for Western blot (immunoblot) analyses and immunofluorescence assays, we identified a 34-kDa virion-associated protein which accumulated in the cytoplasm of infected cells. To confirm that LR1.1 indeed encoded the 34-kDa polypeptide, we inserted a DNA fragment containing circ coding sequences into the Autographa californica baculovirus genome. A group of recombinant polypeptides with sizes of 32, 34, and 35 kDa were identified by their reactivity with the antipeptide serum. Chicken egg yolk antibodies raised against total proteins of insect cells infected with the recombinant baculovirus identified the 34-kDa circ protein specified by BHV-1. The recombinant circ polypeptides and the circ protein specified by BHV-1 were both myristylated, as determined by radiolabeling with [3H]myristic acid. It was noted that the circ gene could be deleted from the BHV-1 genome without impairing virus replication in cell culture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello L. J., Whitbeck J. C., Lawrence W. C. Map location of the thymidine kinase gene of bovine herpesvirus 1. J Virol. 1987 Dec;61(12):4023–4025. doi: 10.1128/jvi.61.12.4023-4025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G. J., Abrams C. C., King A. M., Roosien J., Vlak J. M. Myristoylation of foot-and-mouth disease virus capsid protein precursors is independent of other viral proteins and occurs in both mammalian and insect cells. J Gen Virol. 1991 Mar;72(Pt 3):747–751. doi: 10.1099/0022-1317-72-3-747. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Engels M., Giuliani C., Wild P., Beck T. M., Loepfe E., Wyler R. The genome of bovine herpesvirus 1 (BHV-1) strains exhibiting a neuropathogenic potential compared to known BHV-1 strains by restriction site mapping and cross-hybridization. Virus Res. 1986 Oct;6(1):57–73. doi: 10.1016/0168-1702(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Fraefel C., Wirth U. V., Vogt B., Schwyzer M. Immediate-early transcription over covalently joined genome ends of bovine herpesvirus 1: the circ gene. J Virol. 1993 Mar;67(3):1328–1333. doi: 10.1128/jvi.67.3.1328-1333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraefel C., Zeng J., Choffat Y., Engels M., Schwyzer M., Ackermann M. Identification and zinc dependence of the bovine herpesvirus 1 transactivator protein BICP0. J Virol. 1994 May;68(5):3154–3162. doi: 10.1128/jvi.68.5.3154-3162.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedli K., Metzler A. E. Reactivity of monoclonal antibodies to proteins of a neurotropic bovine herpesvirus 1 (BHV-1) strain and to proteins of representative BHV-1 strains. Arch Virol. 1987;94(1-2):109–122. doi: 10.1007/BF01313729. [DOI] [PubMed] [Google Scholar]
- Gassmann M., Thömmes P., Weiser T., Hübscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990 May;4(8):2528–2532. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harty R. N., Caughman G. B., Holden V. R., O'Callaghan D. J. Characterization of the myristylated polypeptide encoded by the UL1 gene that is conserved in the genome of defective interfering particles of equine herpesvirus 1. J Virol. 1993 Jul;67(7):4122–4132. doi: 10.1128/jvi.67.7.4122-4132.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDRICK J. W., GILLESPIE J. H., MCENTEE K. Infectious pustular vulvovaginitis of cattle. Cornell Vet. 1958 Oct;48(4):458–495. [PubMed] [Google Scholar]
- Lawrence W. C., D'urso R. C., Kundel C. A., Whitbeck J. C., Bello L. J. Map location of the gene for a 130,000-dalton glycoprotein of bovine herpesvirus 1. J Virol. 1986 Nov;60(2):405–414. doi: 10.1128/jvi.60.2.405-414.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean C. A., Clark B., McGeoch D. J. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J Gen Virol. 1989 Dec;70(Pt 12):3147–3157. doi: 10.1099/0022-1317-70-12-3147. [DOI] [PubMed] [Google Scholar]
- MacLean C. A., Dolan A., Jamieson F. E., McGeoch D. J. The myristylated virion proteins of herpes simplex virus type 1: investigation of their role in the virus life cycle. J Gen Virol. 1992 Mar;73(Pt 3):539–547. doi: 10.1099/0022-1317-73-3-539. [DOI] [PubMed] [Google Scholar]
- Metzler A. E., Schudel A. A., Engels M. Bovine herpesvirus 1: molecular and antigenic characteristics of variant viruses isolated from calves with neurological disease. Arch Virol. 1986;87(3-4):205–217. doi: 10.1007/BF01315300. [DOI] [PubMed] [Google Scholar]
- Miller J. M., Whetstone C. A., Bello L. J., Lawrence W. C. Determination of ability of a thymidine kinase-negative deletion mutant of bovine herpesvirus-1 to cause abortion in cattle. Am J Vet Res. 1991 Jul;52(7):1038–1043. [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer M., Vlcek C., Menekse O., Fraefel C., Paces V. Promoter, spliced leader, and coding sequence for BICP4, the largest of the immediate-early proteins of bovine herpesvirus 1. Virology. 1993 Nov;197(1):349–357. doi: 10.1006/viro.1993.1596. [DOI] [PubMed] [Google Scholar]
- Schwyzer M., Wirth U. V., Vogt B., Fraefel C. BICP22 of bovine herpesvirus 1 is encoded by a spliced 1.7 kb RNA which exhibits immediate early and late transcription kinetics. J Gen Virol. 1994 Jul;75(Pt 7):1703–1711. doi: 10.1099/0022-1317-75-7-1703. [DOI] [PubMed] [Google Scholar]
- Wirth U. V., Fraefel C., Vogt B., Vlcek C., Paces V., Schwyzer M. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3' coterminal and encode a putative zinc finger transactivator protein. J Virol. 1992 May;66(5):2763–2772. doi: 10.1128/jvi.66.5.2763-2772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth U. V., Vogt B., Schwyzer M. The three major immediate-early transcripts of bovine herpesvirus 1 arise from two divergent and spliced transcription units. J Virol. 1991 Jan;65(1):195–205. doi: 10.1128/jvi.65.1.195-205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalamanchili R. R., Raengsakulrach B., O'Callaghan D. J. Equine herpesvirus 1 sequence near the left terminus codes for two open reading frames. Virus Res. 1991 Mar;18(2-3):109–116. doi: 10.1016/0168-1702(91)90012-k. [DOI] [PubMed] [Google Scholar]