Abstract
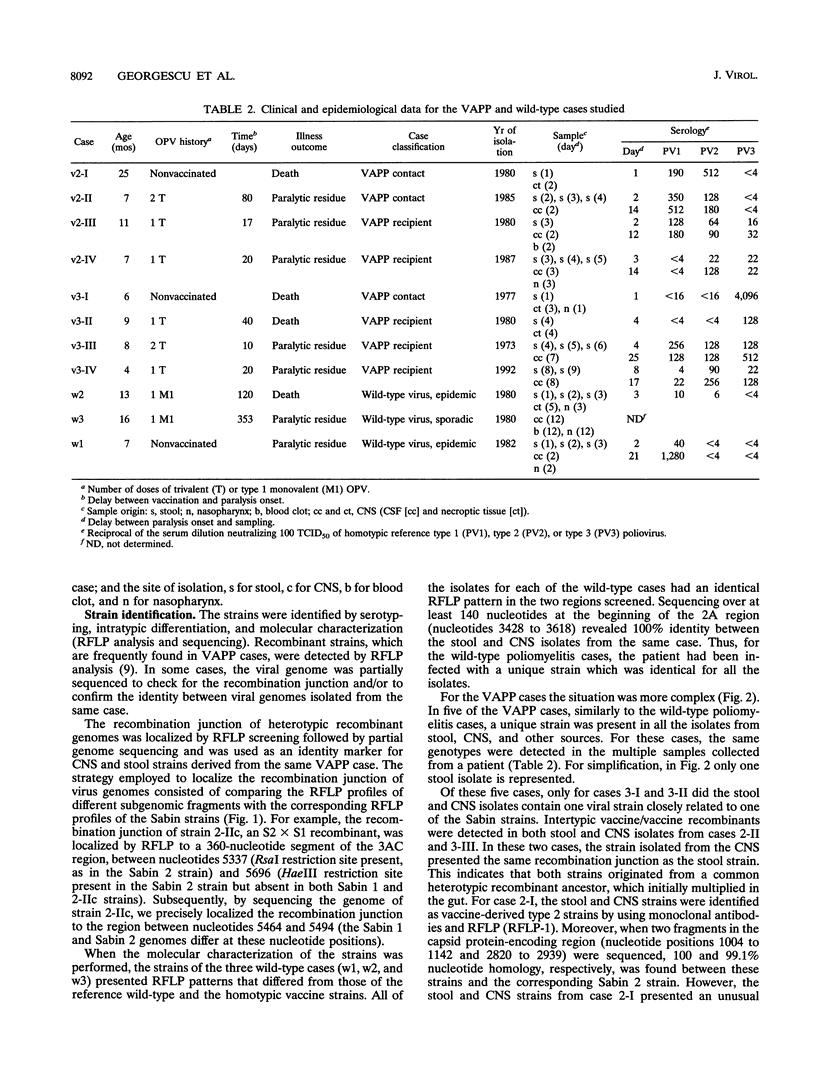
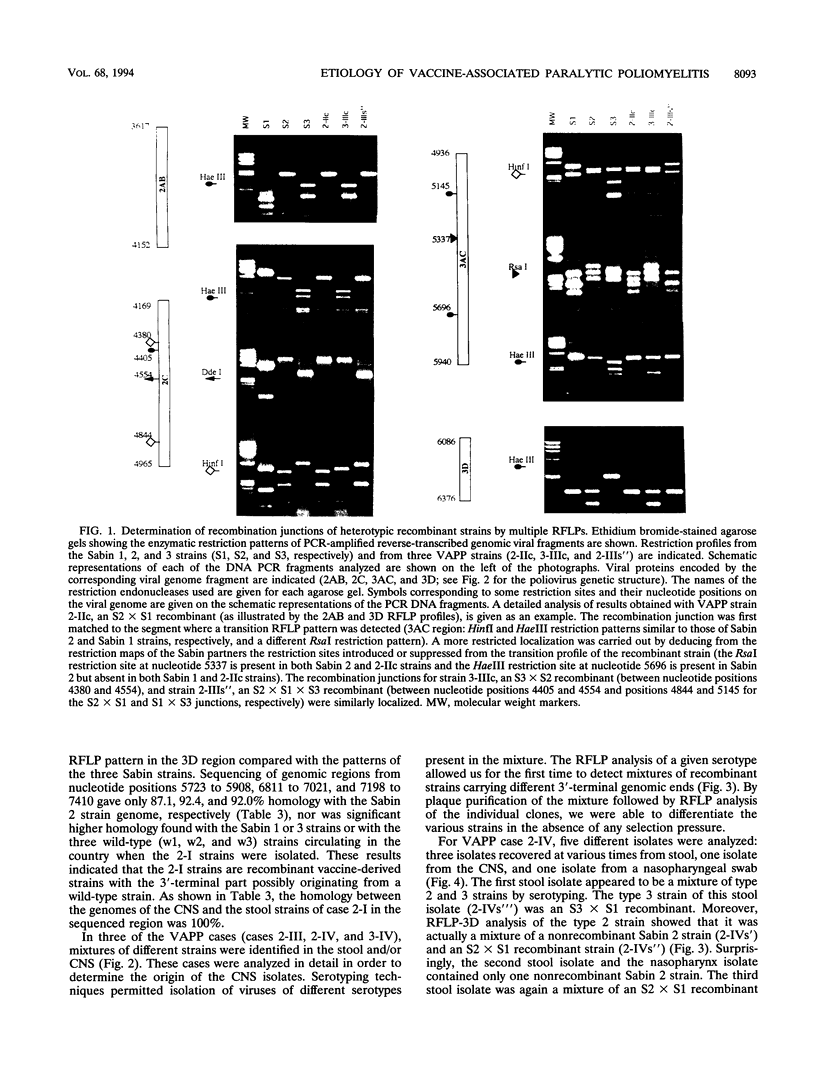
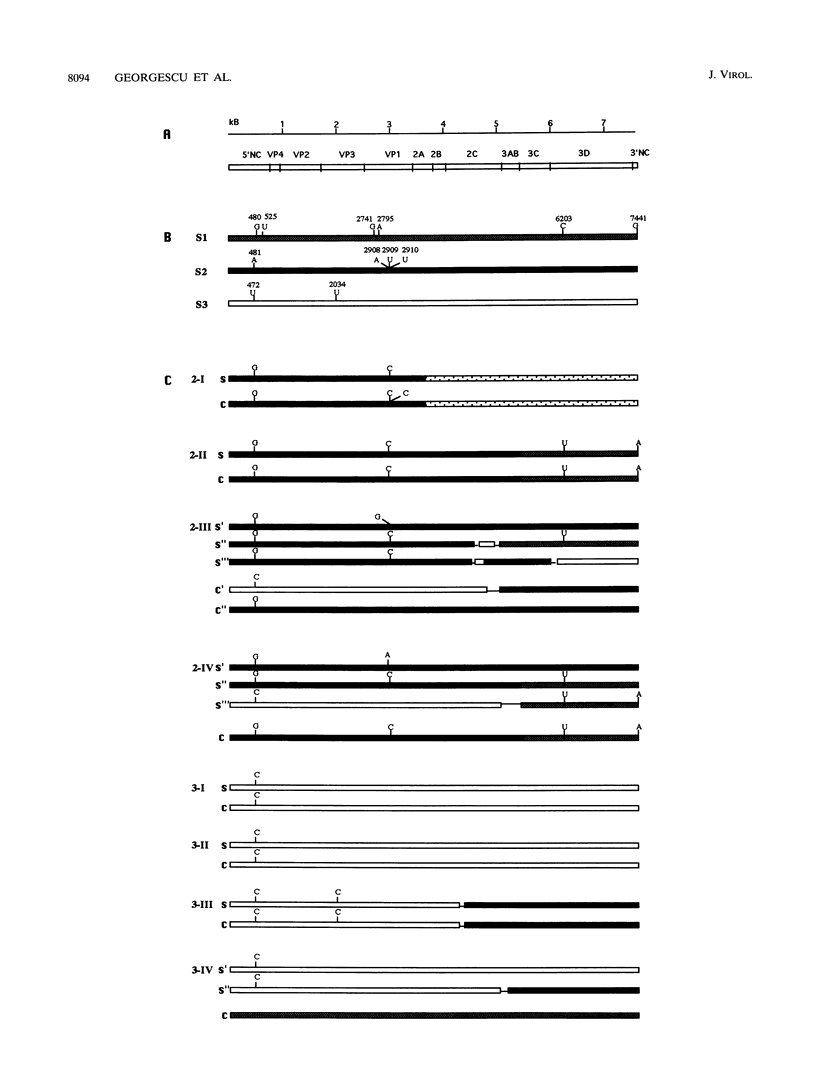
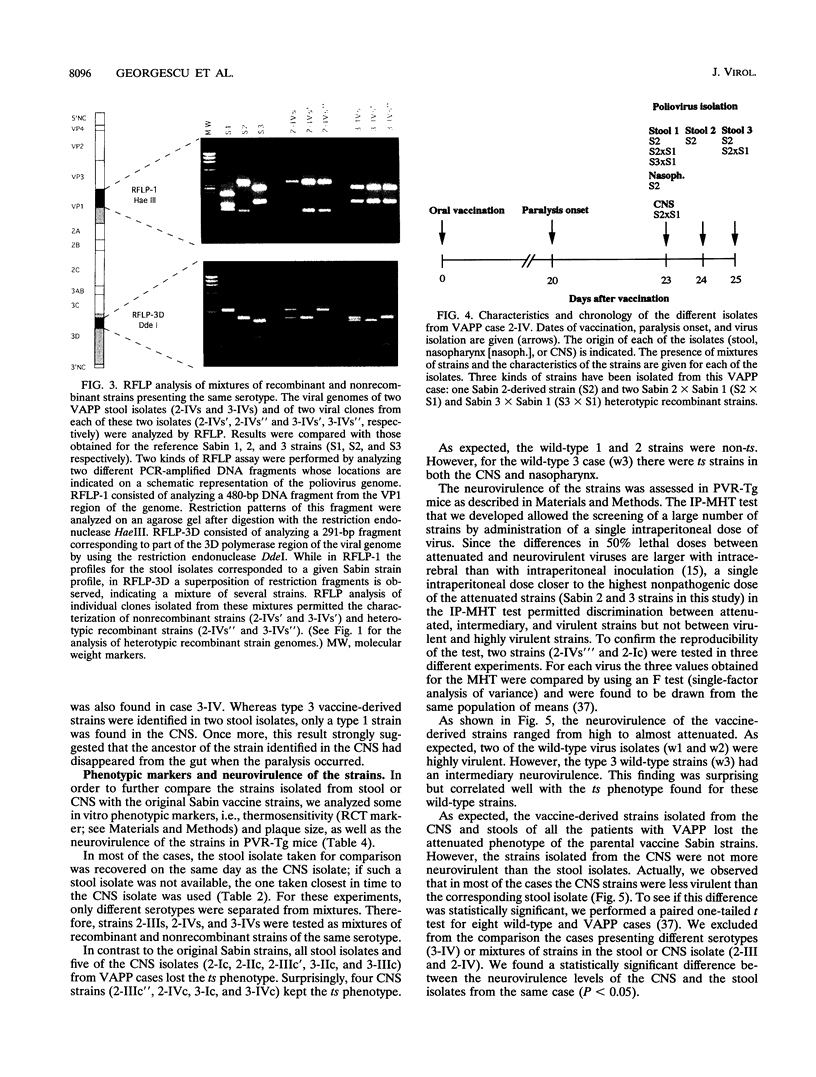
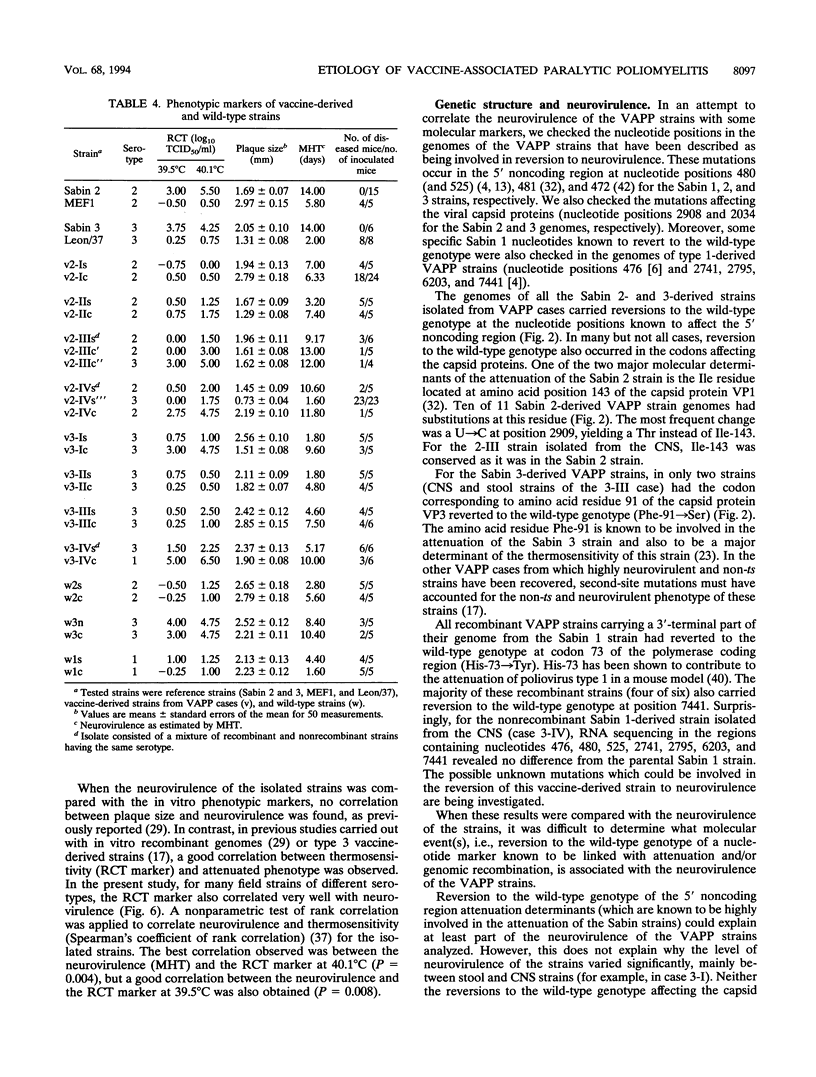
To establish the etiology of vaccine-associated paralytic poliomyelitis (VAPP), isolates from the central nervous system (CNS) from eight patients with VAPP were compared with stool isolates from the same patients. The vaccine (Sabin) origin was checked for all of the available isolates. Unique and similar strains were recovered from paired stool and CNS samples for five of the eight VAPP cases and the three wild-type cases included in the study. In the remaining three VAPP cases, the stool samples and, in one case, the CNS samples contained mixtures of strains. In two of these cases an equivalent of the CNS isolate was found among the strains separated by plaque purification from stool mixtures, and in one case different strains were isolated from CNS and stool. This shows that the stool isolate in VAPP might not be always representative of the etiologic agent of the neurological disease. A wide variety of poliovirus vaccine genomic structures appeared to be implicated in the etiology of VAPP. Of nine CNS vaccine-derived strains, four were nonrecombinant and five were recombinant (vaccine/vaccine or even vaccine/nonvaccine). The neuropathogenic potential of the isolates was evaluated in transgenic mice sensitive to poliovirus. All of the CNS-isolated strains lost the attenuated phenotype of the Sabin strains. However, for half of them, the neurovirulence was lower than expected, suggesting that the degree of neurovirulence for transgenic mice is not necessarily correlated with the neuropathogenicity in humans.
Full text
PDF


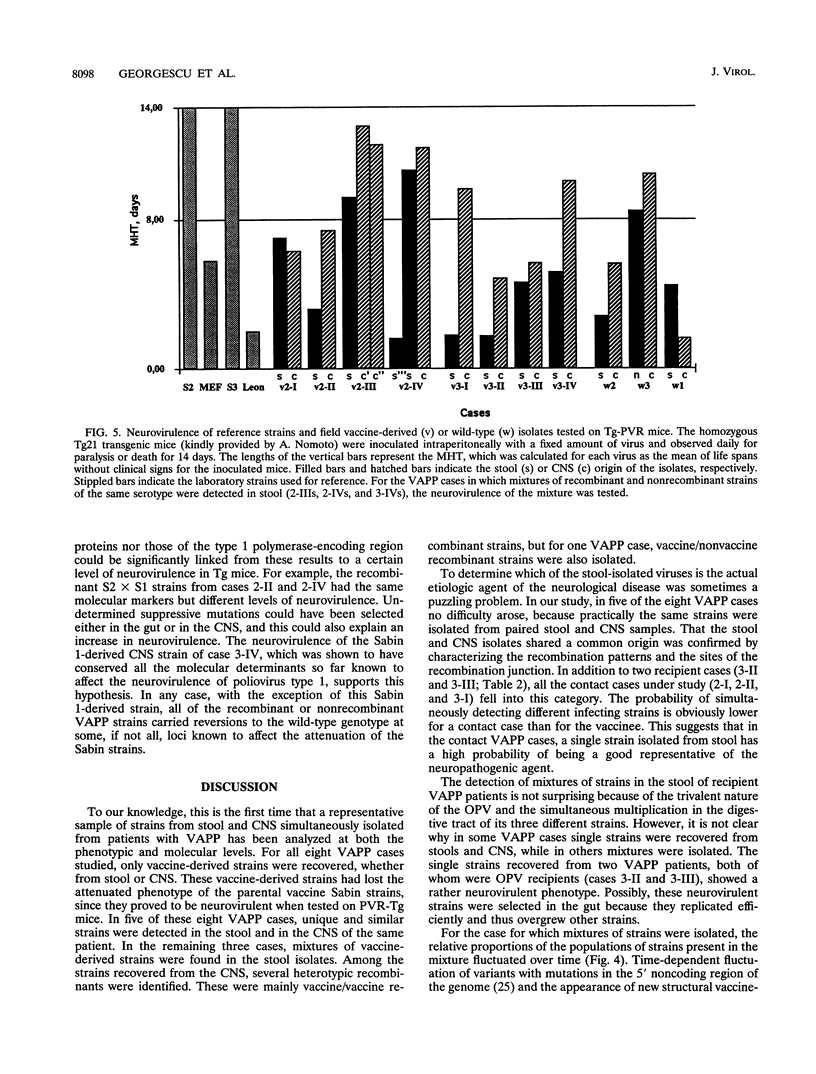


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrus J. K., de Quadros C. A., Olive J. M. The surveillance challenge: final stages of eradication of poliomyelitis in the Americas. MMWR CDC Surveill Summ. 1992 Mar;41(1):21–26. [PubMed] [Google Scholar]
- Balanant J., Guillot S., Candrea A., Delpeyroux F., Crainic R. The natural genomic variability of poliovirus analyzed by a restriction fragment length polymorphism assay. Virology. 1991 Oct;184(2):645–654. doi: 10.1016/0042-6822(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Christodoulou C., Colbere-Garapin F., Macadam A., Taffs L. F., Marsden S., Minor P., Horaud F. Mapping of mutations associated with neurovirulence in monkeys infected with Sabin 1 poliovirus revertants selected at high temperature. J Virol. 1990 Oct;64(10):4922–4929. doi: 10.1128/jvi.64.10.4922-4929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov K. M., Powers L. B., Noonan K. E., Roninson I. B., Levenbook I. S. Correlation between amount of virus with altered nucleotide sequence and the monkey test for acceptability of oral poliovirus vaccine. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):199–203. doi: 10.1073/pnas.88.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras G., Dimock K., Furesz J., Gardell C., Hazlett D., Karpinski K., McCorkle G., Wu L. Genetic characterization of Sabin types 1 and 3 poliovaccine virus following serial passage in the human intestinal tract. Biologicals. 1992 Mar;20(1):15–26. doi: 10.1016/s1045-1056(05)80003-x. [DOI] [PubMed] [Google Scholar]
- Crainic R., Couillin P., Blondel B., Cabau N., Boué A., Horodniceanu F. Natural variation of poliovirus neutralization epitopes. Infect Immun. 1983 Sep;41(3):1217–1225. doi: 10.1128/iai.41.3.1217-1225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichot O., Girard M. An improved method for sequencing of RNA templates. Nucleic Acids Res. 1990 Oct 25;18(20):6162–6162. doi: 10.1093/nar/18.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
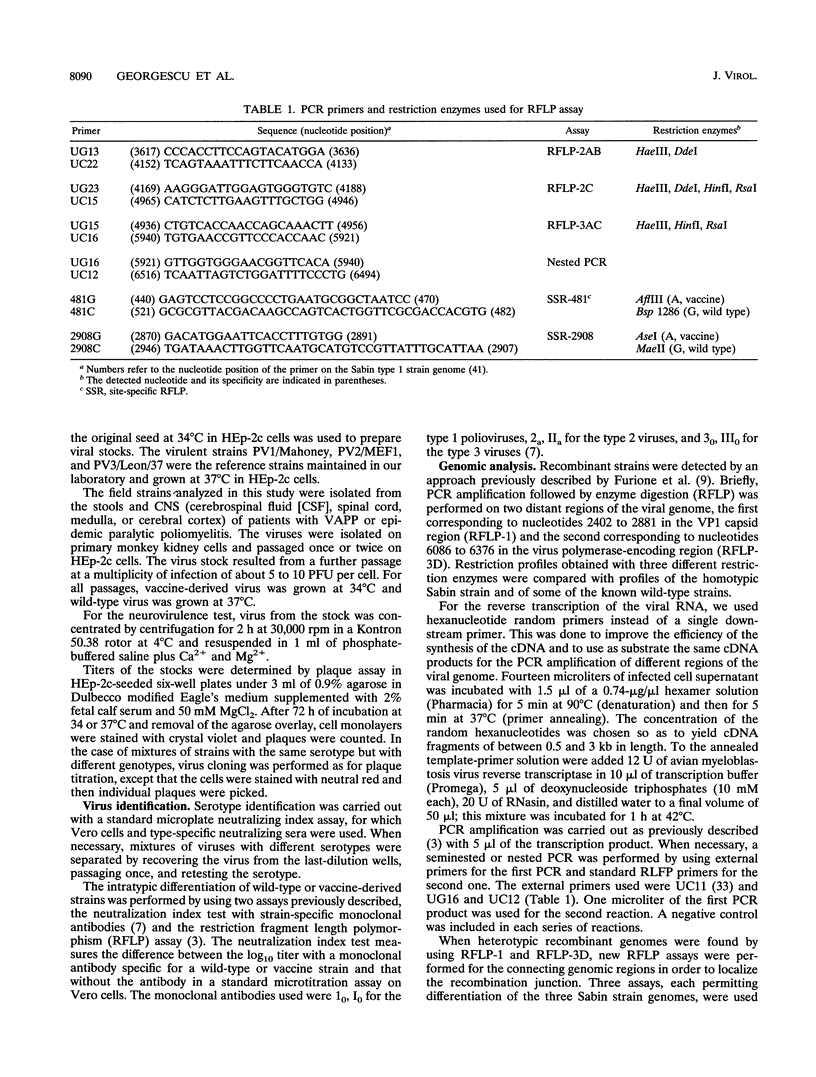
- Furione M., Guillot S., Otelea D., Balanant J., Candrea A., Crainic R. Polioviruses with natural recombinant genomes isolated from vaccine-associated paralytic poliomyelitis. Virology. 1993 Sep;196(1):199–208. doi: 10.1006/viro.1993.1468. [DOI] [PubMed] [Google Scholar]
- Hayward J. C., Gillespie S. M., Kaplan K. M., Packer R., Pallansch M., Plotkin S., Schonberger L. B. Outbreak of poliomyelitis-like paralysis associated with enterovirus 71. Pediatr Infect Dis J. 1989 Sep;8(9):611–616. doi: 10.1097/00006454-198909000-00009. [DOI] [PubMed] [Google Scholar]
- Horie H., Koike S., Kurata T., Sato-Yoshida Y., Ise I., Ota Y., Abe S., Hioki K., Kato H., Taya C. Transgenic mice carrying the human poliovirus receptor: new animal models for study of poliovirus neurovirulence. J Virol. 1994 Feb;68(2):681–688. doi: 10.1128/jvi.68.2.681-688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura N., Kohara M., Abe S., Komatsu T., Tago K., Arita M., Nomoto A. Determinants in the 5' noncoding region of poliovirus Sabin 1 RNA that influence the attenuation phenotype. J Virol. 1989 Mar;63(3):1302–1309. doi: 10.1128/jvi.63.3.1302-1309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Horie H., Sato Y., Ise I., Taya C., Nomura T., Yoshioka I., Yonekawa H., Nomoto A. Poliovirus-sensitive transgenic mice as a new animal model. Dev Biol Stand. 1993;78:101–107. [PubMed] [Google Scholar]
- Koike S., Taya C., Kurata T., Abe S., Ise I., Yonekawa H., Nomoto A. Transgenic mice susceptible to poliovirus. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):951–955. doi: 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macadam A. J., Arnold C., Howlett J., John A., Marsden S., Taffs F., Reeve P., Hamada N., Wareham K., Almond J. Reversion of the attenuated and temperature-sensitive phenotypes of the Sabin type 3 strain of poliovirus in vaccinees. Virology. 1989 Oct;172(2):408–414. doi: 10.1016/0042-6822(89)90183-9. [DOI] [PubMed] [Google Scholar]
- Martin A., Benichou D., Couderc T., Hogle J. M., Wychowski C., Van der Werf S., Girard M. Use of type 1/type 2 chimeric polioviruses to study determinants of poliovirus type 1 neurovirulence in a mouse model. Virology. 1991 Feb;180(2):648–658. doi: 10.1016/0042-6822(91)90078-p. [DOI] [PubMed] [Google Scholar]
- Melnick J. L. Enterovirus type 71 infections: a varied clinical pattern sometimes mimicking paralytic poliomyelitis. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S387–S390. doi: 10.1093/clinids/6.supplement_2.s387. [DOI] [PubMed] [Google Scholar]
- Melnick J. L. The discovery of the enteroviruses and the classification of poliovirus among them. Biologicals. 1993 Dec;21(4):305–309. doi: 10.1006/biol.1993.1088. [DOI] [PubMed] [Google Scholar]
- Minor P. D. Attenuation and reversion of the Sabin vaccine strains of poliovirus. Dev Biol Stand. 1993;78:17–26. [PubMed] [Google Scholar]
- Minor P. D., Dunn G., Evans D. M., Magrath D. I., John A., Howlett J., Phillips A., Westrop G., Wareham K., Almond J. W. The temperature sensitivity of the Sabin type 3 vaccine strain of poliovirus: molecular and structural effects of a mutation in the capsid protein VP3. J Gen Virol. 1989 May;70(Pt 5):1117–1123. doi: 10.1099/0022-1317-70-5-1117. [DOI] [PubMed] [Google Scholar]
- Minor P. D., John A., Ferguson M., Icenogle J. P. Antigenic and molecular evolution of the vaccine strain of type 3 poliovirus during the period of excretion by a primary vaccinee. J Gen Virol. 1986 Apr;67(Pt 4):693–706. doi: 10.1099/0022-1317-67-4-693. [DOI] [PubMed] [Google Scholar]
- Muzychenko A. R., Lipskaya GYu, Maslova S. V., Svitkin Y. V., Pilipenko E. V., Nottay B. K., Kew O. M., Agol V. I. Coupled mutations in the 5'-untranslated region of the Sabin poliovirus strains during in vivo passages: structural and functional implications. Virus Res. 1991 Oct;21(2):111–122. doi: 10.1016/0168-1702(91)90002-d. [DOI] [PubMed] [Google Scholar]
- Nakano J. H., Hatch M. H., Thieme M. L., Nottay B. Parameters for differentiating vaccine-derived and wild poliovirus strains. Prog Med Virol. 1978;24:178–206. [PubMed] [Google Scholar]
- Nkowane B. M., Wassilak S. G., Orenstein W. A., Bart K. J., Schonberger L. B., Hinman A. R., Kew O. M. Vaccine-associated paralytic poliomyelitis. United States: 1973 through 1984. JAMA. 1987 Mar 13;257(10):1335–1340. [PubMed] [Google Scholar]
- Nomoto A. Recombinant polioviruses as candidates for oral live poliovaccines. Microbiol Immunol. 1993;37(3):169–174. doi: 10.1111/j.1348-0421.1993.tb03196.x. [DOI] [PubMed] [Google Scholar]
- Omata T., Kohara M., Kuge S., Komatsu T., Abe S., Semler B. L., Kameda A., Itoh H., Arita M., Wimmer E. Genetic analysis of the attenuation phenotype of poliovirus type 1. J Virol. 1986 May;58(2):348–358. doi: 10.1128/jvi.58.2.348-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otelea D., Guillot S., Furione M., Combiescu A. A., Balanant J., Candrea A., Crainic R. Genomic modifications in naturally occurring neurovirulent revertants of Sabin 1 polioviruses. Dev Biol Stand. 1993;78:33–38. [PubMed] [Google Scholar]
- Ren R. B., Costantini F., Gorgacz E. J., Lee J. J., Racaniello V. R. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell. 1990 Oct 19;63(2):353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- Ren R. B., Moss E. G., Racaniello V. R. Identification of two determinants that attenuate vaccine-related type 2 poliovirus. J Virol. 1991 Mar;65(3):1377–1382. doi: 10.1128/jvi.65.3.1377-1382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Hesse R., Pallansch M. A., Nottay B. K., Kew O. M. Geographic distribution of wild poliovirus type 1 genotypes. Virology. 1987 Oct;160(2):311–322. doi: 10.1016/0042-6822(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Sabin A. B. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985 Mar;151(3):420–436. doi: 10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- Sutter R. W., Brink E. W., Cochi S. L., Kew O. M., Orenstein W. A., Biellik R. J., Hinman A. R. A new epidemiologic and laboratory classification system for paralytic poliomyelitis cases. Am J Public Health. 1989 Apr;79(4):495–498. doi: 10.2105/ajph.79.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy-Panit M., Blondel B., Martin A., Tekaia F., Horaud F., Delpeyroux F. A mutation in the RNA polymerase of poliovirus type 1 contributes to attenuation in mice. J Virol. 1993 Aug;67(8):4630–4638. doi: 10.1128/jvi.67.8.4630-4638.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Westrop G. D., Wareham K. A., Evans D. M., Dunn G., Minor P. D., Magrath D. I., Taffs F., Marsden S., Skinner M. A., Schild G. C. Genetic basis of attenuation of the Sabin type 3 oral poliovirus vaccine. J Virol. 1989 Mar;63(3):1338–1344. doi: 10.1128/jvi.63.3.1338-1344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]