Abstract
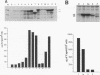
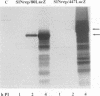
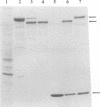
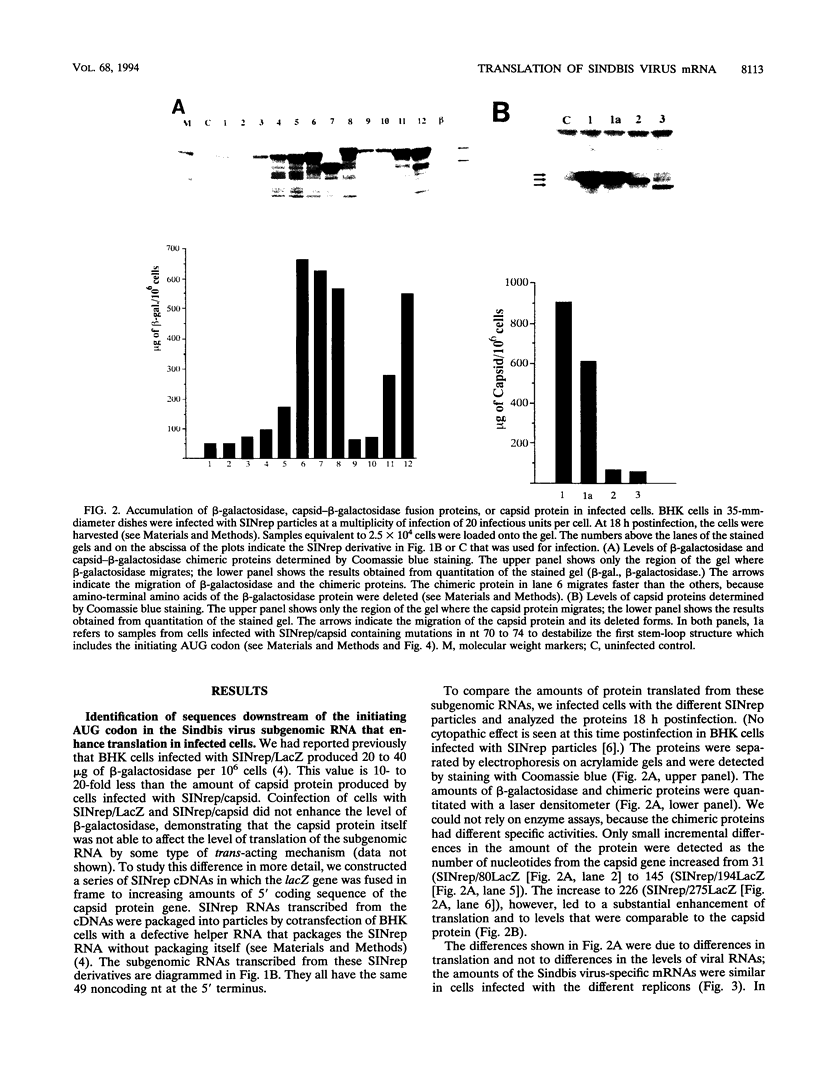
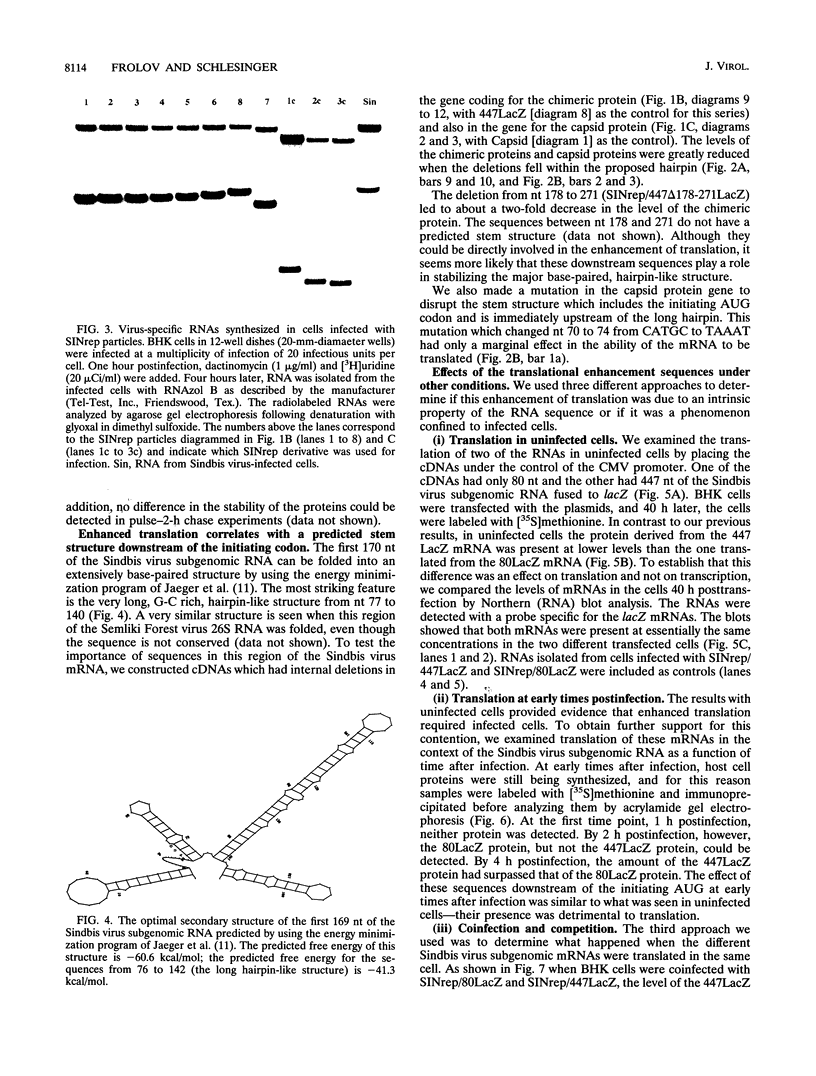
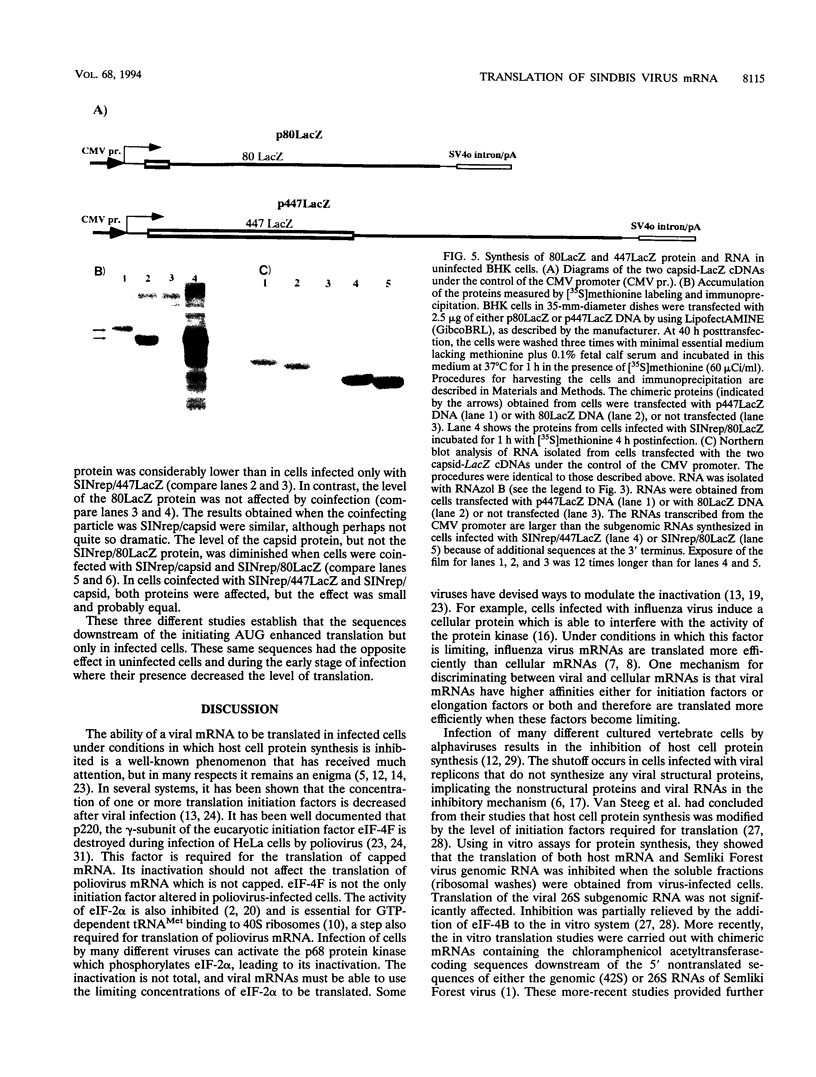
One incentive for developing the alphavirus Sindbis virus as a vector for the expression of heterologous proteins is the very high level of viral structural proteins that accumulates in infected cells. Although replacement of the structural protein genes by a heterologous gene should lead to an equivalent accumulation of the heterologous protein, the Sindbis virus capsid protein is produced at a level 10- to 20-fold higher than that of any foreign protein. Chimeric mRNAs which contain the first 275 nucleotides of the Sindbis virus 26S mRNA fused to the lacZ gene are also translated at the higher level. The enhancing sequences, located downstream of the AUG codon that initiates translation of the capsid protein, have a predicted hairpin-like structure; deletions in this region destroy the activity. These sequences enhance translation in infected cells but have the opposite effect in uninfected cells. Furthermore, translation of this RNA in infected cells is suppressed by a second viral RNA lacking the hairpin-like structure, but translation of the latter RNA is not affected. We propose that the hairpin-like structure presents a barrier to the movement of the ribosomes during translation of mRNA. In infected cells, under conditions in which this mRNA is essentially the only RNA being translated, a slowdown in the transit of the ribosomes gives factors present at low concentrations a chance to bind to the translation complex and permits a high level of functional complexes to be formed. In uninfected cells and in infected cells translating two different viral subgenomic mRNAs, a pause in the movement of the ribosomes along the RNA is no longer an advantage, because the required factors are now usurped by other translation complexes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berben-Bloemheuvel G., Kasperaitis M. A., van Heugten H., Thomas A. A., van Steeg H., Voorma H. O. Interaction of initiation factors with the cap structure of chimaeric mRNA containing the 5'-untranslated regions of Semliki Forest virus RNA is related to translational efficiency. Eur J Biochem. 1992 Sep 15;208(3):581–587. doi: 10.1111/j.1432-1033.1992.tb17222.x. [DOI] [PubMed] [Google Scholar]
- Black T. L., Safer B., Hovanessian A., Katze M. G. The cellular 68,000-Mr protein kinase is highly autophosphorylated and activated yet significantly degraded during poliovirus infection: implications for translational regulation. J Virol. 1989 May;63(5):2244–2251. doi: 10.1128/jvi.63.5.2244-2251.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenbeek P. J., Frolov I., Rice C. M., Schlesinger S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol. 1993 Nov;67(11):6439–6446. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov I., Schlesinger S. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J Virol. 1994 Mar;68(3):1721–1727. doi: 10.1128/jvi.68.3.1721-1727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel M. S., Katze M. G. Translational control by influenza virus. Selective and cap-dependent translation of viral mRNAs in infected cells. J Biol Chem. 1992 May 5;267(13):9383–9390. [PubMed] [Google Scholar]
- Garfinkel M. S., Katze M. G. Translational control by influenza virus. Selective translation is mediated by sequences within the viral mRNA 5'-untranslated region. J Biol Chem. 1993 Oct 25;268(30):22223–22226. [PubMed] [Google Scholar]
- Geigenmüller-Gnirke U., Nitschko H., Schlesinger S. Deletion analysis of the capsid protein of Sindbis virus: identification of the RNA binding region. J Virol. 1993 Mar;67(3):1620–1626. doi: 10.1128/jvi.67.3.1620-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8301–8305. doi: 10.1073/pnas.87.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Ranki M. Inhibition of cell functions by RNA-virus infections. Annu Rev Microbiol. 1984;38:91–109. doi: 10.1146/annurev.mi.38.100184.000515. [DOI] [PubMed] [Google Scholar]
- Lee T. G., Tomita J., Hovanessian A. G., Katze M. G. Characterization and regulation of the 58,000-dalton cellular inhibitor of the interferon-induced, dsRNA-activated protein kinase. J Biol Chem. 1992 Jul 15;267(20):14238–14243. [PubMed] [Google Scholar]
- Liljeström P., Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991 Dec;9(12):1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Lusa S., Huylebroeck D., Garoff H. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J Virol. 1991 Aug;65(8):4107–4113. doi: 10.1128/jvi.65.8.4107-4113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. E., Racaniello V. R. Inhibition of translation in cells infected with a poliovirus 2Apro mutant correlates with phosphorylation of the alpha subunit of eucaryotic initiation factor 2. J Virol. 1989 Dec;63(12):5069–5075. doi: 10.1128/jvi.63.12.5069-5075.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S. Alphaviruses--vectors for the expression of heterologous genes. Trends Biotechnol. 1993 Jan;11(1):18–22. doi: 10.1016/0167-7799(93)90070-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Measures and countermeasures in the modulation of initiation factor activities by viruses. New Biol. 1990 May;2(5):402–409. [PubMed] [Google Scholar]
- Svitkin Y. V., Agol V. I. Translational barrier in central region of encephalomyocarditis virus genome. Modulation by elongation factor 2 (eEF-2). Eur J Biochem. 1983 Jun 1;133(1):145–154. doi: 10.1111/j.1432-1033.1983.tb07440.x. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988 Nov;7(11):3559–3569. doi: 10.1002/j.1460-2075.1988.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C., Levis R., Shen P., Schlesinger S., Rice C. M., Huang H. V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989 Mar 3;243(4895):1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- van Steeg H., Thomas A., Verbeek S., Kasperaitis M., Voorma H. O., Benne R. Shutoff of neuroblastoma cell protein synthesis by Semliki Forest virus: loss of ability of crude initiation factors to recognize early Semliki Forest virus and host mRNA's. J Virol. 1981 May;38(2):728–736. doi: 10.1128/jvi.38.2.728-736.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steeg H., van Grinsven M., van Mansfeld F., Voorma H. O., Benne R. Initiation of protein synthesis in neuroblastoma cells infected by Semliki Forest Virus. A decreased requirement of late viral mRNA for eIF-4B and cap binding protein. FEBS Lett. 1981 Jun 29;129(1):62–66. doi: 10.1016/0014-5793(81)80756-9. [DOI] [PubMed] [Google Scholar]