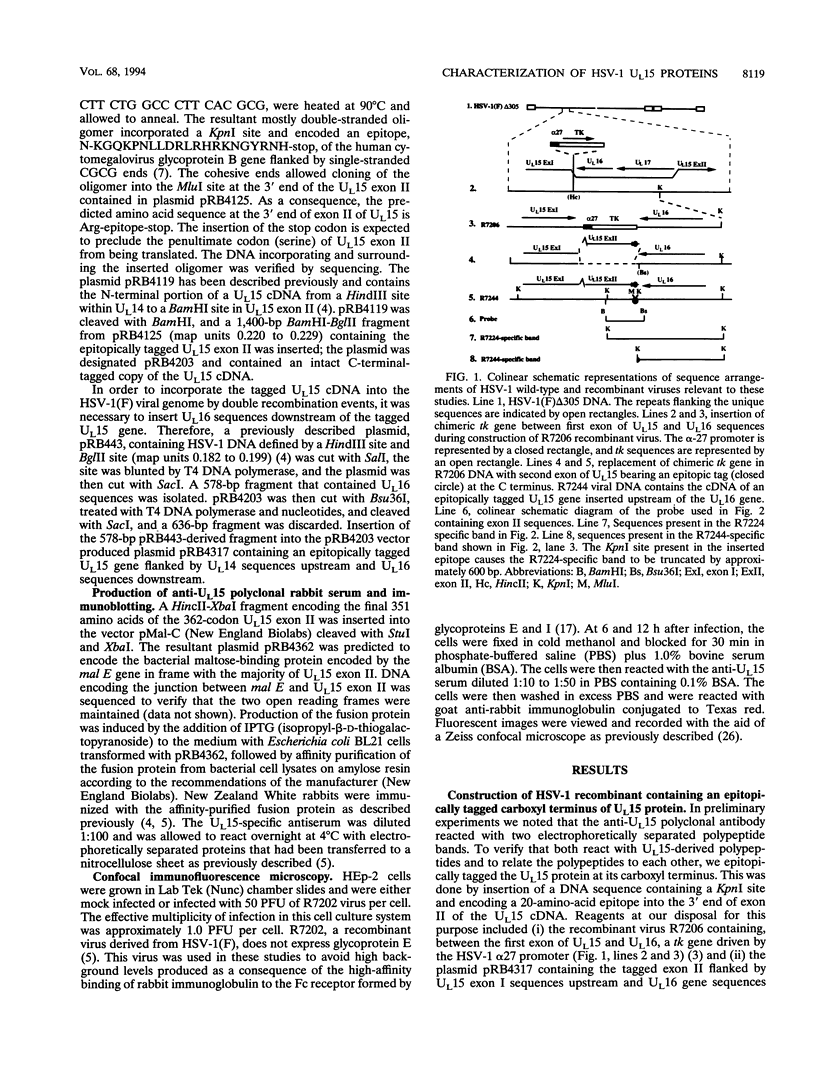
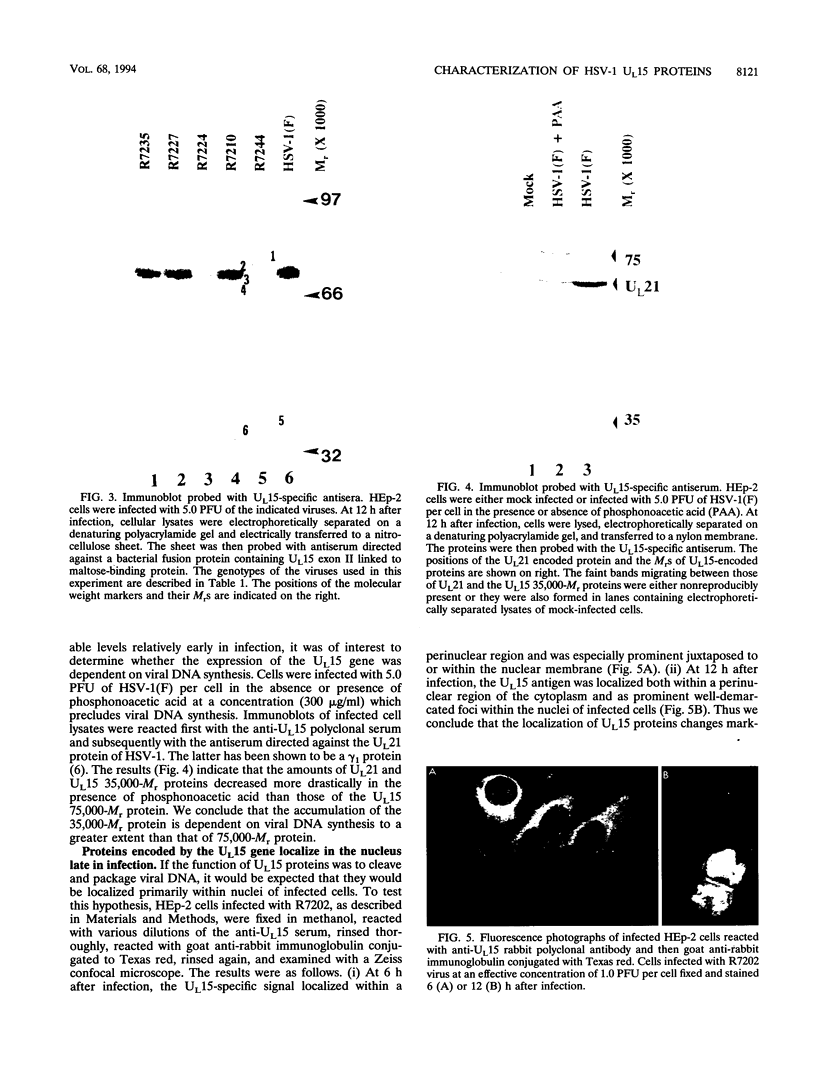
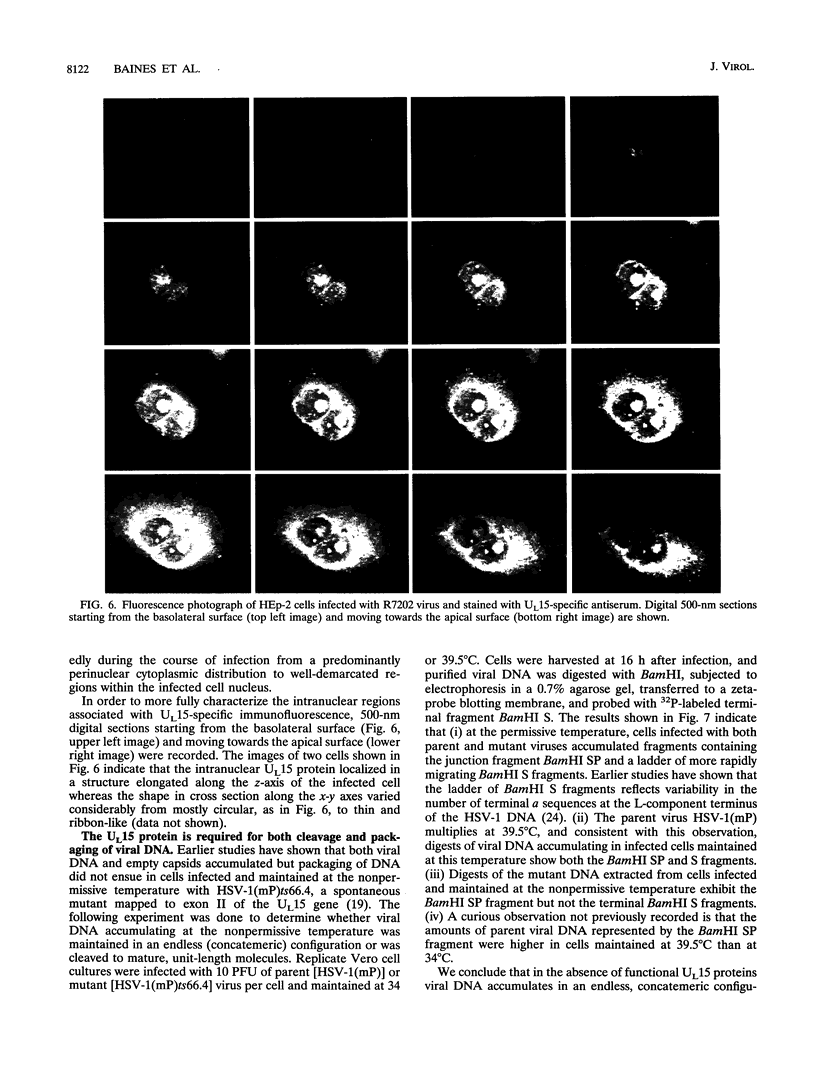
Abstract
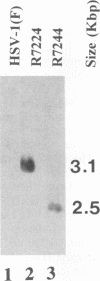
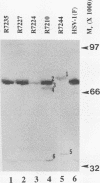
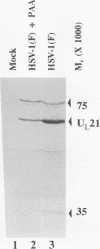
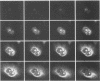
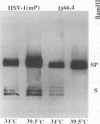
Previous studies have shown that a ts mutant [herpes simplex virus 1 (mP)ts66.4] in the UL15 gene fails to package viral DNA into capsids (A. P. W. Poon and B. Roizman, J. Virol. 67:4497-4503, 1993) and that although the intron separating the first and second exons of the UL15 gene contains UL16 and UL17 open reading frames, replacement of the first exon with a cDNA copy of the entire gene does not affect viral replication (J.D. Baines, and B. Roizman, J. Virol. 66:5621-5626, 1992). We report that (i) a polyclonal rabbit antiserum generated against a chimeric protein consisting of the bacterial maltose-binding protein fused in frame to the majority of sequences contained in the second exon of the UL15 gene reacted with two proteins with M(r) of 35,000 and 75,000, respectively, in cells infected with a virus containing the authentic gene yielding a spliced mRNA or with a virus in which the authentic UL15 gene was replaced with a cDNA copy. (ii) Insertion of 20 additional codons into the C terminus of UL15 exon II caused a reduction in the electrophoretic mobility of both the apparently 35,000- and 75,000-M(r) proteins, unambiguously demonstrating that both share the carboxyl terminus of the UL15 exon II. (iii) Accumulation of the 35,000-M(r) protein was reduced in cells infected and maintained in the presence of phosphonoacetate, an inhibitor of viral DNA synthesis. (iv) The UL15 proteins were localized in the perinuclear space at 6 h after infection and largely in the nucleus at 12 h after infection. (v) Viral DNA accumulating in cells infected with herpes simplex virus 1(mP)ts66.4 and maintained at the nonpermissive temperature was in an endless (concatemeric) form, and therefore UL15 is required for the cleavage of mature, unit-length molecules for packaging into capsids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines J. D., Koyama A. H., Huang T., Roizman B. The UL21 gene products of herpes simplex virus 1 are dispensable for growth in cultured cells. J Virol. 1994 May;68(5):2929–2936. doi: 10.1128/jvi.68.5.2929-2936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993 Mar;67(3):1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The cDNA of UL15, a highly conserved herpes simplex virus 1 gene, effectively replaces the two exons of the wild-type virus. J Virol. 1992 Sep;66(9):5621–5626. doi: 10.1128/jvi.66.9.5621-5626.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J Virol. 1991 Feb;65(2):938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basgoz N., Qadri I., Navarro D., Sears A., Lennette E., Youngblom J., Pereira L. The amino terminus of human cytomegalovirus glycoprotein B contains epitopes that vary among strains. J Gen Virol. 1992 Apr;73(Pt 4):983–988. doi: 10.1099/0022-1317-73-4-983. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. P., Rao V. B. A novel terminase activity associated with the DNA packaging protein gp17 of bacteriophage T4. Virology. 1993 Sep;196(1):34–44. doi: 10.1006/viro.1993.1452. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Draper K. G., Kelly T. J., Wagner E. K. An unusual spliced herpes simplex virus type 1 transcript with sequence homology to Epstein-Barr virus DNA. J Virol. 1985 May;54(2):317–328. doi: 10.1128/jvi.54.2.317-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J. Channel catfish virus: a new type of herpesvirus. Virology. 1992 Jan;186(1):9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Poon A. P., Roizman B. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J Virol. 1993 Aug;67(8):4497–4503. doi: 10.1128/jvi.67.8.4497-4503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Sherman G., Bachenheimer S. L. DNA processing in temperature-sensitive morphogenic mutants of HSV-1. Virology. 1987 Jun;158(2):427–430. doi: 10.1016/0042-6822(87)90214-5. [DOI] [PubMed] [Google Scholar]
- Tengelsen L. A., Pederson N. E., Shaver P. R., Wathen M. W., Homa F. L. Herpes simplex virus type 1 DNA cleavage and encapsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J Virol. 1993 Jun;67(6):3470–3480. doi: 10.1128/jvi.67.6.3470-3480.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognon M., Cassai E., Rotola A., Roizman B. The heterogenous regions in herpes simplex virus 1 DNA. Microbiologica. 1983 Jul;6(3):191–198. [PubMed] [Google Scholar]
- Wagner M. J., Summers W. C. Structure of the joint region and the termini of the DNA of herpes simplex virus type 1. J Virol. 1978 Aug;27(2):374–387. doi: 10.1128/jvi.27.2.374-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Ward P. L., Campadelli-Fiume G., Avitabile E., Roizman B. Localization and putative function of the UL20 membrane protein in cells infected with herpes simplex virus 1. J Virol. 1994 Nov;68(11):7406–7417. doi: 10.1128/jvi.68.11.7406-7417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Carmichael E. P., Aschman D. P., Goldstein D. J., Schaffer P. A. Genetic and phenotypic characterization of mutants in four essential genes that map to the left half of HSV-1 UL DNA. Virology. 1987 Nov;161(1):198–210. doi: 10.1016/0042-6822(87)90186-3. [DOI] [PubMed] [Google Scholar]
- Worrad D. M., Caradonna S. The herpes simplex virus type 2 UL3 open reading frame encodes a nuclear localizing phosphoprotein. Virology. 1993 Aug;195(2):364–376. doi: 10.1006/viro.1993.1386. [DOI] [PubMed] [Google Scholar]
- al-Kobaisi M. F., Rixon F. J., McDougall I., Preston V. G. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology. 1991 Jan;180(1):380–388. doi: 10.1016/0042-6822(91)90043-b. [DOI] [PubMed] [Google Scholar]
- de Bruyn Kops A., Knipe D. M. Preexisting nuclear architecture defines the intranuclear location of herpesvirus DNA replication structures. J Virol. 1994 Jun;68(6):3512–3526. doi: 10.1128/jvi.68.6.3512-3526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]