Abstract
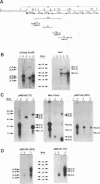
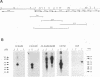
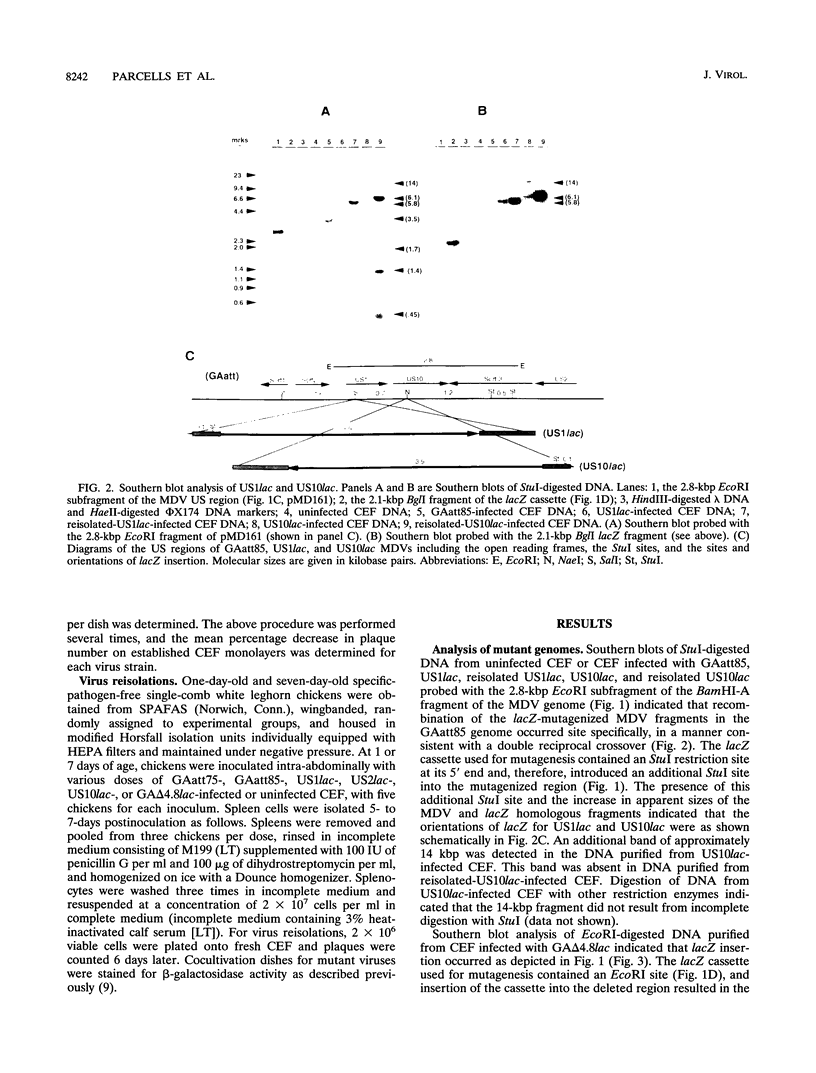
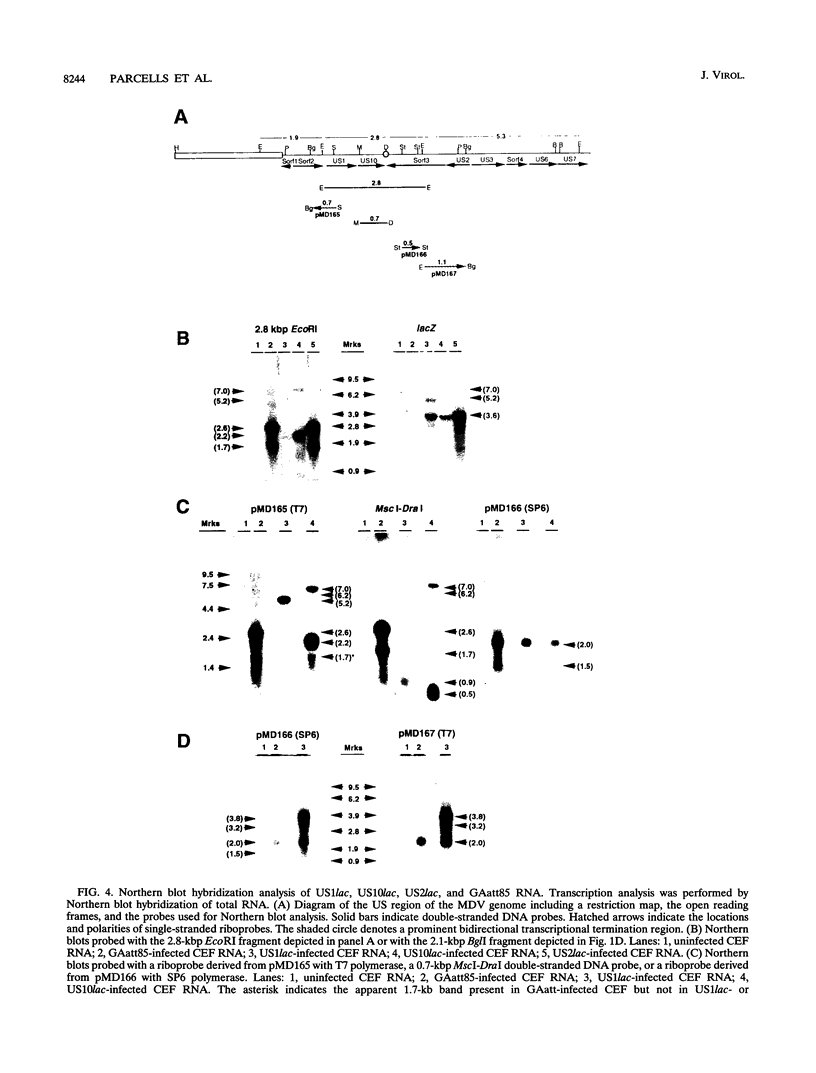
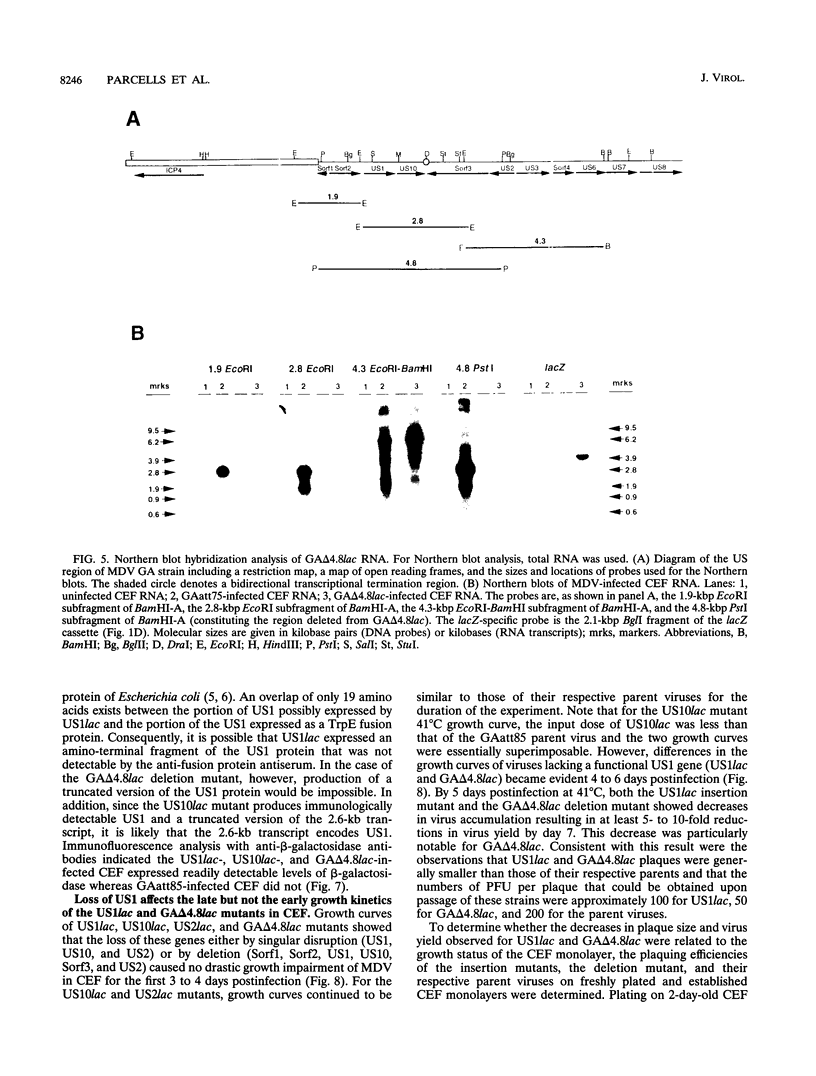
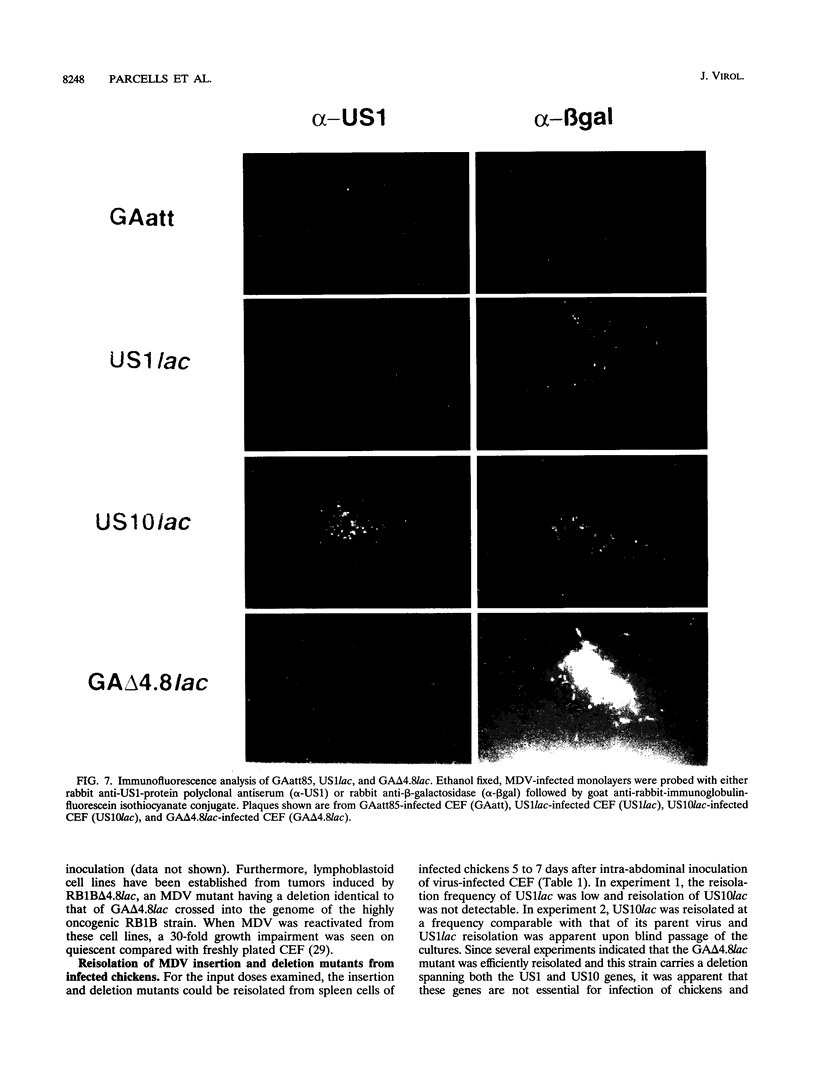
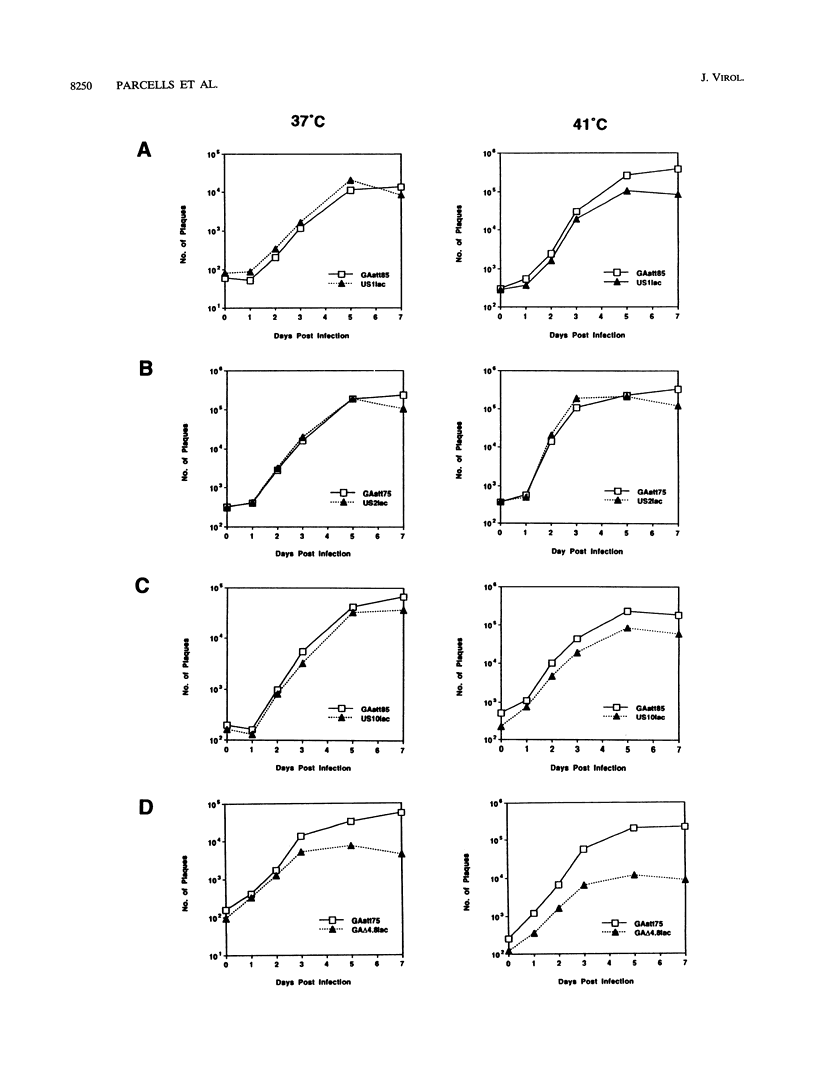
We report the characterization of Marek's disease virus (MDV) strains having mutations in various genes that map to the unique short (US) region of the viral genome. A deletion mutant (GA delta 4.8lac) lacks 4.8 kbp of US region DNA, the deleted segment having been replaced by the lacZ gene of Escherichia coli. This deletion results in the loss of the MDV-encoded US1, US10, and US2 homologs of herpes simplex virus type 1, as well as three putative MDV-specific genes, Sorf1, Sorf2, and Sorf3. Two mutants containing lacZ insertions in the US1 and US10 genes have been constructed, and we have previously reported a US2lac insertion mutant (J. L. Cantello, A. S. Anderson, A. Francesconi, and R. W. Morgan, J. Virol. 65:1584-1588, 1991). The isolation of these mutants indicates that the relevant genes are not required for growth of MDV in chicken embryo fibroblasts. The mutants had early growth kinetics indistinguishable from those of their parent viruses; however, 5 to 7 days after being plated, the US1 insertion mutant (US1lac) and the GA delta 4.8lac deletion mutant showed a 5- to 10-fold decrease in virus growth. This decrease in virus accumulation correlated with a 30 to 50% decrease in plaquing efficiency when these viruses were plated onto established versus fresh chicken embryo fibroblast monolayers compared with a 10 to 15% decrease seen for the parent viruses and for the US10lac or US2lac insertion mutants. Finally, GA delta 4.8lac could be reisolated from chickens, indicating that the deleted genes are not required for the infection of chickens following intra-abdominal inoculation of an attenuated serotype 1 MDV.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Sarmiento M., Roizman B. Application of antibody to synthetic peptides for characterization of the intact and truncated alpha 22 protein specified by herpes simplex virus 1 and the R325 alpha 22- deletion mutant. J Virol. 1985 Oct;56(1):207–215. doi: 10.1128/jvi.56.1.207-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho J. A., Mitchell C., Roizman B. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J Virol. 1993 Jul;67(7):3891–3900. doi: 10.1128/jvi.67.7.3891-3900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Harland J. Three mutants of herpes simplex virus type 2: one lacking the genes US10, US11 and US12 and two in which Rs has been extended by 6 kb to 0.91 map units with loss of Us sequences between 0.94 and the Us/TRs junction. J Gen Virol. 1987 Jan;68(Pt 1):1–18. doi: 10.1099/0022-1317-68-1-1. [DOI] [PubMed] [Google Scholar]
- Buckmaster A. E., Scott S. D., Sanderson M. J., Boursnell M. E., Ross N. L., Binns M. M. Gene sequence and mapping data from Marek's disease virus and herpesvirus of turkeys: implications for herpesvirus classification. J Gen Virol. 1988 Aug;69(Pt 8):2033–2042. doi: 10.1099/0022-1317-69-8-2033. [DOI] [PubMed] [Google Scholar]
- Cantello J. L., Anderson A. S., Francesconi A., Morgan R. W. Isolation of a Marek's disease virus (MDV) recombinant containing the lacZ gene of Escherichia coli stably inserted within the MDV US2 gene. J Virol. 1991 Mar;65(3):1584–1588. doi: 10.1128/jvi.65.3.1584-1588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinane A. A., Rixon F. J., Davison A. J. Characterization of the genome of equine herpesvirus 1 subtype 2. J Gen Virol. 1988 Jul;69(Pt 7):1575–1590. doi: 10.1099/0022-1317-69-7-1575. [DOI] [PubMed] [Google Scholar]
- Davison A. J. DNA sequence of the US component of the varicella-zoster virus genome. EMBO J. 1983;2(12):2203–2209. doi: 10.1002/j.1460-2075.1983.tb01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J., Petkevich J. M. On the association of virus proteins with the nuclei of cells infected with herpes simplex virus. J Gen Virol. 1978 Jun;39(3):519–529. doi: 10.1099/0022-1317-39-3-519. [DOI] [PubMed] [Google Scholar]
- Fenwick M., Walker M., Marshall L. Some characteristics of an early protein (ICP 22) synthesized in cells infected with herpes simplex virus. J Gen Virol. 1980 Apr;47(2):333–341. doi: 10.1099/0022-1317-47-2-333. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulou U., Michaelidou A., Roizman B., Mavromara-Nazos P. Identification of a new transcriptional unit that yields a gene product within the unique sequences of the short component of the herpes simplex virus 1 genome. J Virol. 1993 Jul;67(7):3961–3968. doi: 10.1128/jvi.67.7.3961-3968.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden V. R., Yalamanchili R. R., Harty R. N., O'Callaghan D. J. ICP22 homolog of equine herpesvirus 1: expression from early and late promoters. J Virol. 1992 Feb;66(2):664–673. doi: 10.1128/jvi.66.2.664-673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden V. R., Yalamanchili R. R., Harty R. N., O'Callaghan D. J. Identification and characterization of an equine herpesvirus 1 late gene encoding a potential zinc finger. Virology. 1992 Jun;188(2):704–713. doi: 10.1016/0042-6822(92)90525-t. [DOI] [PubMed] [Google Scholar]
- Jackers P., Defechereux P., Baudoux L., Lambert C., Massaer M., Merville-Louis M. P., Rentier B., Piette J. Characterization of regulatory functions of the varicella-zoster virus gene 63-encoded protein. J Virol. 1992 Jun;66(6):3899–3903. doi: 10.1128/jvi.66.6.3899-3903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987 May 1;236(4801):573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986 May;58(2):583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Moss H. W., McNab D., Frame M. C. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J Gen Virol. 1987 Jan;68(Pt 1):19–38. doi: 10.1099/0022-1317-68-1-19. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. The genomes of the human herpesviruses: contents, relationships, and evolution. Annu Rev Microbiol. 1989;43:235–265. doi: 10.1146/annurev.mi.43.100189.001315. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T., Kaplan A. S. Role of glycoprotein gIII of pseudorabies virus in virulence. J Virol. 1988 Aug;62(8):2712–2717. doi: 10.1128/jvi.62.8.2712-2717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. W., Cantello J. L., McDermott C. H. Transfection of chicken embryo fibroblasts with Marek's disease virus DNA. Avian Dis. 1990 Apr-Jun;34(2):345–351. [PubMed] [Google Scholar]
- Poffenberger K. L., Raichlen P. E., Herman R. C. In vitro characterization of a herpes simplex virus type 1 ICP22 deletion mutant. Virus Genes. 1993 Jun;7(2):171–186. doi: 10.1007/BF01702397. [DOI] [PubMed] [Google Scholar]
- Post L. E., Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981 Jul;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross L. J., Binns M. M., Pastorek J. DNA sequence and organization of genes in a 5.5 kbp EcoRI fragment mapping in the short unique segment of Marek's disease virus (strain RB1B). J Gen Virol. 1991 Apr;72(Pt 4):949–954. doi: 10.1099/0022-1317-72-4-949. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Urakawa T., Hirayama Y., Miki N., Yamamoto M., Hirai K. Sequence determination and genetic content of an 8.9-kb restriction fragment in the short unique region and the internal inverted repeat of Marek's disease virus type 1 DNA. Virus Genes. 1992 Nov;6(4):365–378. doi: 10.1007/BF01703085. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Urakawa T., Hirayama Y., Miki N., Yamamoto M., Zhu G. S., Hirai K. Marek's disease virus protein kinase gene identified within the short unique region of the viral genome is not essential for viral replication in cell culture and vaccine-induced immunity in chickens. Virology. 1993 Jul;195(1):140–148. doi: 10.1006/viro.1993.1354. [DOI] [PubMed] [Google Scholar]
- Schat K. A., Buckmaster A., Ross L. J. Partial transcription map of Marek's disease herpesvirus in lytically infected cells and lymphoblastoid cell lines. Int J Cancer. 1989 Jul 15;44(1):101–109. doi: 10.1002/ijc.2910440119. [DOI] [PubMed] [Google Scholar]
- Schwyzer M., Vlcek C., Menekse O., Fraefel C., Paces V. Promoter, spliced leader, and coding sequence for BICP4, the largest of the immediate-early proteins of bovine herpesvirus 1. Virology. 1993 Nov;197(1):349–357. doi: 10.1006/viro.1993.1596. [DOI] [PubMed] [Google Scholar]
- Sears A. E., Halliburton I. W., Meignier B., Silver S., Roizman B. Herpes simplex virus 1 mutant deleted in the alpha 22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985 Aug;55(2):338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermeijer P. J., Claessens J. A., Jenniskens P. E., Mockett A. P., Thijssen R. A., Willemse M. J., Morgan R. W. Avian herpesvirus as a live viral vector for the expression of heterologous antigens. Vaccine. 1993;11(3):349–358. doi: 10.1016/0264-410x(93)90198-7. [DOI] [PubMed] [Google Scholar]
- Telford E. A., Watson M. S., McBride K., Davison A. J. The DNA sequence of equine herpesvirus-1. Virology. 1992 Jul;189(1):304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Umene K. Conversion of a fraction of the unique sequence to part of the inverted repeats in the S component of the herpes simplex virus type 1 genome. J Gen Virol. 1986 Jun;67(Pt 6):1035–1048. doi: 10.1099/0022-1317-67-6-1035. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Levine M., Glorioso J. C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987 May 1;236(4801):576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- Whitton J. L., Clements J. B. The junctions between the repetitive and the short unique sequences of the herpes simplex virus genome are determined by the polypeptide-coding regions of two spliced immediate-early mRNAs. J Gen Virol. 1984 Mar;65(Pt 3):451–466. doi: 10.1099/0022-1317-65-3-451. [DOI] [PubMed] [Google Scholar]
- Zelník V., Darteil R., Audonnet J. C., Smith G. D., Riviere M., Pastorek J., Ross L. J. The complete sequence and gene organization of the short unique region of herpesvirus of turkeys. J Gen Virol. 1993 Oct;74(Pt 10):2151–2162. doi: 10.1099/0022-1317-74-10-2151. [DOI] [PubMed] [Google Scholar]
- Zhang G., Leader D. P. The structure of the pseudorabies virus genome at the end of the inverted repeat sequences proximal to the junction with the short unique region. J Gen Virol. 1990 Oct;71(Pt 10):2433–2441. doi: 10.1099/0022-1317-71-10-2433. [DOI] [PubMed] [Google Scholar]
- de Wind N., Zijderveld A., Glazenburg K., Gielkens A., Berns A. Linker insertion mutagenesis of herpesviruses: inactivation of single genes within the Us region of pseudorabies virus. J Virol. 1990 Oct;64(10):4691–4696. doi: 10.1128/jvi.64.10.4691-4696.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl M., van der Gulden H., de Wind N., Gielkens A., Berns A. Identification of two genes in the unique short region of pseudorabies virus; comparison with herpes simplex virus and varicella-zoster virus. J Gen Virol. 1990 Aug;71(Pt 8):1747–1755. doi: 10.1099/0022-1317-71-8-1747. [DOI] [PubMed] [Google Scholar]