Abstract
Intracellular immunization is an anti-viral gene therapy strategy based on the introduction of DNA templates into cells to stably express genetic elements which inhibit viral gene expression and replication. We have recently developed an intracellular immunization strategy for human immunodeficiency virus (HIV) infection that uses RNA decoys. RNA decoys are short RNA oligonucleotides corresponding to the HIV trans activation response element (TAR) or Rev response element (RRE) sequences, which function by inhibiting the binding of the HIV regulatory proteins Tat and Rev to the authentic HIV RNA TAR and RRE regions, respectively. In this report we describe the characterization of potent RRE decoys containing the minimal 13-nucleotide primary Rev binding domain of the RRE. Using an improved tRNA cassette to express high levels of RRE transcripts in CEM cells, we found that this new generation of minimal RRE decoys were more potent inhibitors of HIV in isolated cell lines than previously described TAR or RRE decoys. CEM cells expressing RRE decoys exhibited diminished Rev function in cotransfection assays, confirming the specificity of inhibition of HIV by RRE decoys and indicating that the 13-nucleotide minimal Rev binding domain defined by using in vitro binding studies also binds Rev in vivo. Significant differences in the degree of HIV inhibition between individual CEM cell lines transduced with RRE decoy vectors which were not due to sequence alterations in the tRNA-RRE DNA template, differences in RRE decoy expression level, or endogenous variations in the resistance of CEM clonal cell lines to HIV were observed. In order to evaluate the efficacy of RRE decoys in a more realistic fashion than by comparison of individual clonal cell lines, polyclonal populations of transduced CEM cells were infected with HIV. By using a novel flow cytometric method for quantitating intracellular p24 expression, one version of the RRE decoys tested in this study was found to be capable of durably protecting polyclonal populations of CEM cells from HIV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeniyi-Jones S., Romeo P. H., Zasloff M. Generation of long read-through transcripts in vivo and in vitro by deletion of 3' termination and processing sequences in the human tRNAimet gene. Nucleic Acids Res. 1984 Jan 25;12(2):1101–1115. doi: 10.1093/nar/12.2.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988 Sep 29;335(6189):395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Bartel D. P., Zapp M. L., Green M. R., Szostak J. W. HIV-1 Rev regulation involves recognition of non-Watson-Crick base pairs in viral RNA. Cell. 1991 Nov 1;67(3):529–536. doi: 10.1016/0092-8674(91)90527-6. [DOI] [PubMed] [Google Scholar]
- Bevec D., Dobrovnik M., Hauber J., Böhnlein E. Inhibition of human immunodeficiency virus type 1 replication in human T cells by retroviral-mediated gene transfer of a dominant-negative Rev trans-activator. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9870–9874. doi: 10.1073/pnas.89.20.9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevec D., Volc-Platzer B., Zimmermann K., Dobrovnik M., Hauber J., Veres G., Böhnlein E. Constitutive expression of chimeric neo-Rev response element transcripts suppresses HIV-1 replication in human CD4+ T lymphocytes. Hum Gene Ther. 1994 Feb;5(2):193–201. doi: 10.1089/hum.1994.5.2-193. [DOI] [PubMed] [Google Scholar]
- Cheong C., Varani G., Tinoco I., Jr Solution structure of an unusually stable RNA hairpin, 5'GGAC(UUCG)GUCC. Nature. 1990 Aug 16;346(6285):680–682. doi: 10.1038/346680a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cochrane A. W., Chen C. H., Rosen C. A. Specific interaction of the human immunodeficiency virus Rev protein with a structured region in the env mRNA. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1198–1202. doi: 10.1073/pnas.87.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. S., Fisk G. J., Hauber J., Usman N., Daly T. J., Rusche J. R. Characterization of HIV-1 REV protein: binding stoichiometry and minimal RNA substrate. Nucleic Acids Res. 1991 Apr 11;19(7):1577–1583. doi: 10.1093/nar/19.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Greene W. C. Regulatory pathways governing HIV-1 replication. Cell. 1989 Aug 11;58(3):423–426. doi: 10.1016/0092-8674(89)90420-0. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Mechanism of action of regulatory proteins encoded by complex retroviruses. Microbiol Rev. 1992 Sep;56(3):375–394. doi: 10.1128/mr.56.3.375-394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L., Bagasra O., Laughlin M. A., Oakes J. W., Pomerantz R. J. Potent inhibition of human immunodeficiency virus type 1 replication by an intracellular anti-Rev single-chain antibody. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5075–5079. doi: 10.1073/pnas.91.11.5075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gatignol A., Buckler-White A., Berkhout B., Jeang K. T. Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science. 1991 Mar 29;251(5001):1597–1600. doi: 10.1126/science.2011739. [DOI] [PubMed] [Google Scholar]
- Gaynor R., Soultanakis E., Kuwabara M., Garcia J., Sigman D. S. Specific binding of a HeLa cell nuclear protein to RNA sequences in the human immunodeficiency virus transactivating region. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4858–4862. doi: 10.1073/pnas.86.13.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Smith C. Gene therapy for infectious diseases: the AIDS model. Trends Genet. 1994 Apr;10(4):139–144. doi: 10.1016/0168-9525(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Graham G. J., Maio J. J. RNA transcripts of the human immunodeficiency virus transactivation response element can inhibit action of the viral transactivator. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5817–5821. doi: 10.1073/pnas.87.15.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantzopoulos P. A., Sullenger B. A., Ungers G., Gilboa E. Improved gene expression upon transfer of the adenosine deaminase minigene outside the transcriptional unit of a retroviral vector. Proc Natl Acad Sci U S A. 1989 May;86(10):3519–3523. doi: 10.1073/pnas.86.10.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy S., Finch J. T., Gait M. J., Karn J., Singh M. Human immunodeficiency virus type 1 regulator of virion expression, rev, forms nucleoprotein filaments after binding to a purine-rich "bubble" located within the rev-responsive region of viral mRNAs. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7366–7370. doi: 10.1073/pnas.88.16.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S. M., Ahmad N., Maitra R. K., Wingfield P., Venkatesan S. Human immunodeficiency virus rev protein recognizes a target sequence in rev-responsive element RNA within the context of RNA secondary structure. J Virol. 1990 Dec;64(12):5966–5975. doi: 10.1128/jvi.64.12.5966-5975.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope T. J., Huang X. J., McDonald D., Parslow T. G. Steroid-receptor fusion of the human immunodeficiency virus type 1 Rev transactivator: mapping cryptic functions of the arginine-rich motif. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7787–7791. doi: 10.1073/pnas.87.19.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. J., Hope T. J., Bond B. L., McDonald D., Grahl K., Parslow T. G. Minimal Rev-response element for type 1 human immunodeficiency virus. J Virol. 1991 Apr;65(4):2131–2134. doi: 10.1128/jvi.65.4.2131-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Improved predictions of secondary structures for RNA. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7706–7710. doi: 10.1073/pnas.86.20.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems J., Brown M., Chang D. D., Sharp P. A. Structural analysis of the interaction between the human immunodeficiency virus Rev protein and the Rev response element. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):683–687. doi: 10.1073/pnas.88.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S. Y., Malim M. H., Cullen B. R., Maizel J. V. A highly conserved RNA folding region coincident with the Rev response element of primate immunodeficiency viruses. Nucleic Acids Res. 1990 Mar 25;18(6):1613–1623. doi: 10.1093/nar/18.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
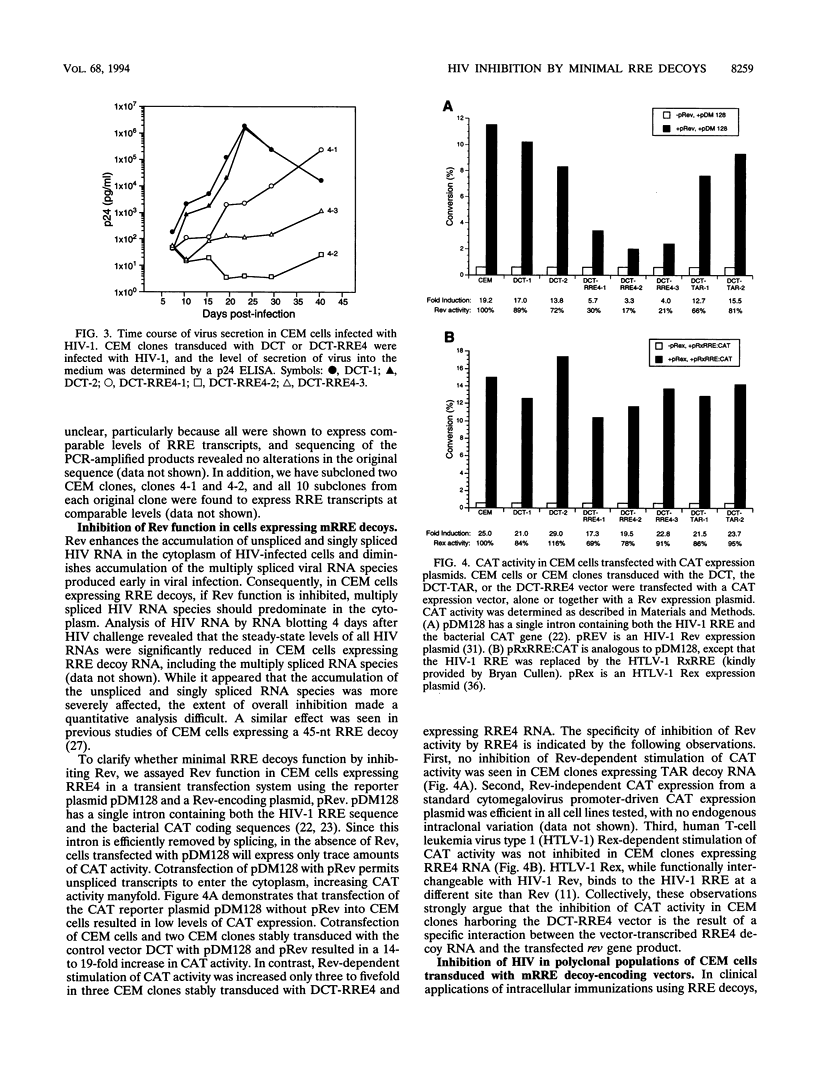
- Lee T. C., Sullenger B. A., Gallardo H. F., Ungers G. E., Gilboa E. Overexpression of RRE-derived sequences inhibits HIV-1 replication in CEM cells. New Biol. 1992 Jan;4(1):66–74. [PubMed] [Google Scholar]
- Lisziewicz J., Rappaport J., Dhar R. Tat-regulated production of multimerized TAR RNA inhibits HIV-1 gene expression. New Biol. 1991 Jan;3(1):82–89. [PubMed] [Google Scholar]
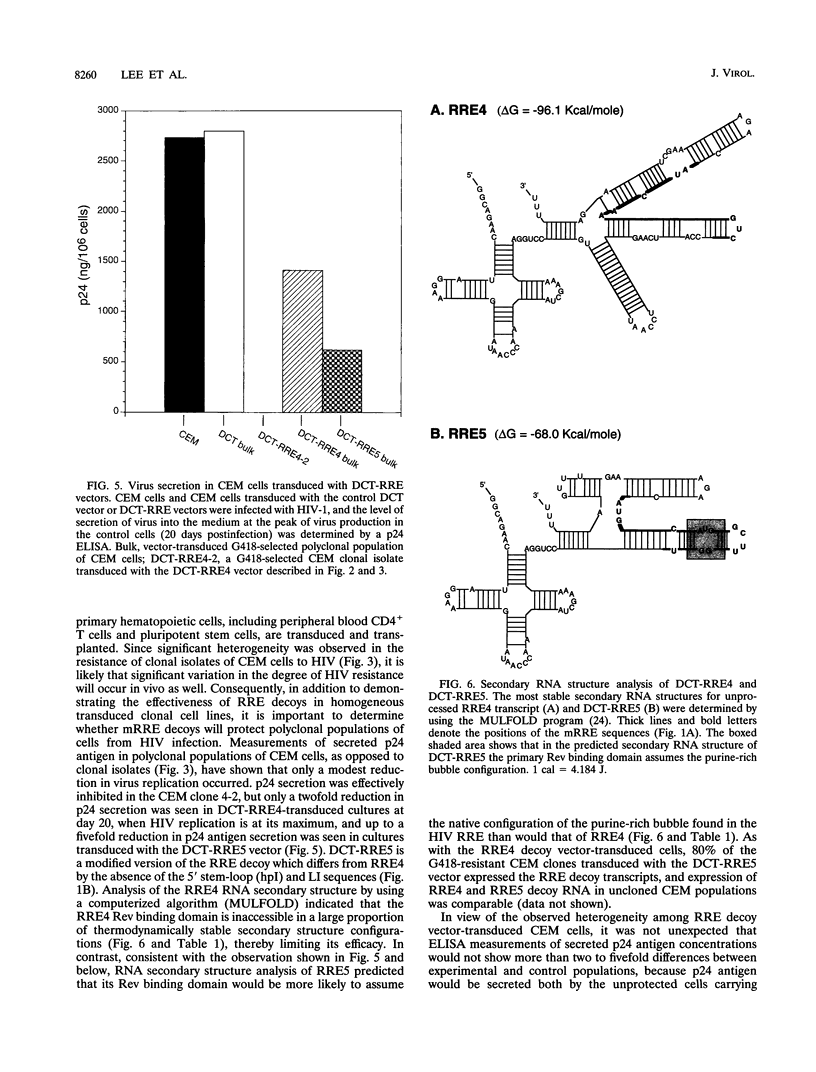
- Liu J., Woffendin C., Yang Z. Y., Nabel G. J. Regulated expression of a dominant negative form of Rev improves resistance to HIV replication in T cells. Gene Ther. 1994 Jan;1(1):32–37. [PubMed] [Google Scholar]
- Malim M. H., Freimuth W. W., Liu J., Boyle T. J., Lyerly H. K., Cullen B. R., Nabel G. J. Stable expression of transdominant Rev protein in human T cells inhibits human immunodeficiency virus replication. J Exp Med. 1992 Oct 1;176(4):1197–1201. doi: 10.1084/jem.176.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Fenrick R., Cullen B. R. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988 Sep 8;335(6186):181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Tiley L. S., McCarn D. F., Rusche J. R., Hauber J., Cullen B. R. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell. 1990 Feb 23;60(4):675–683. doi: 10.1016/0092-8674(90)90670-a. [DOI] [PubMed] [Google Scholar]
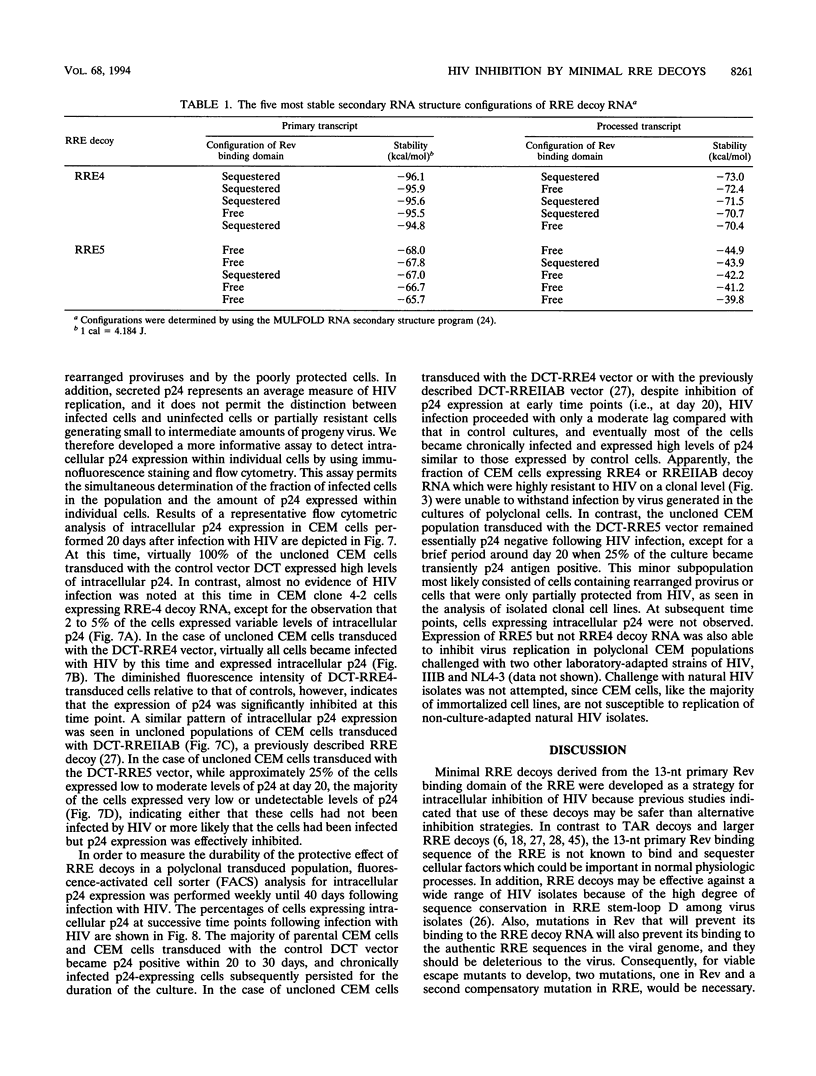
- Marciniak R. A., Garcia-Blanco M. A., Sharp P. A. Identification and characterization of a HeLa nuclear protein that specifically binds to the trans-activation-response (TAR) element of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 May;87(9):3624–3628. doi: 10.1073/pnas.87.9.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P. L., Fischinger P. J. Quantitative infectivity assay for HIV-1 and-2. Nature. 1988 Mar 31;332(6163):469–470. doi: 10.1038/332469a0. [DOI] [PubMed] [Google Scholar]
- Olsen H. S., Nelbock P., Cochrane A. W., Rosen C. A. Secondary structure is the major determinant for interaction of HIV rev protein with RNA. Science. 1990 Feb 16;247(4944):845–848. doi: 10.1126/science.2406903. [DOI] [PubMed] [Google Scholar]
- Rimsky L., Hauber J., Dukovich M., Malim M. H., Langlois A., Cullen B. R., Greene W. C. Functional replacement of the HIV-1 rev protein by the HTLV-1 rex protein. Nature. 1988 Oct 20;335(6192):738–740. doi: 10.1038/335738a0. [DOI] [PubMed] [Google Scholar]
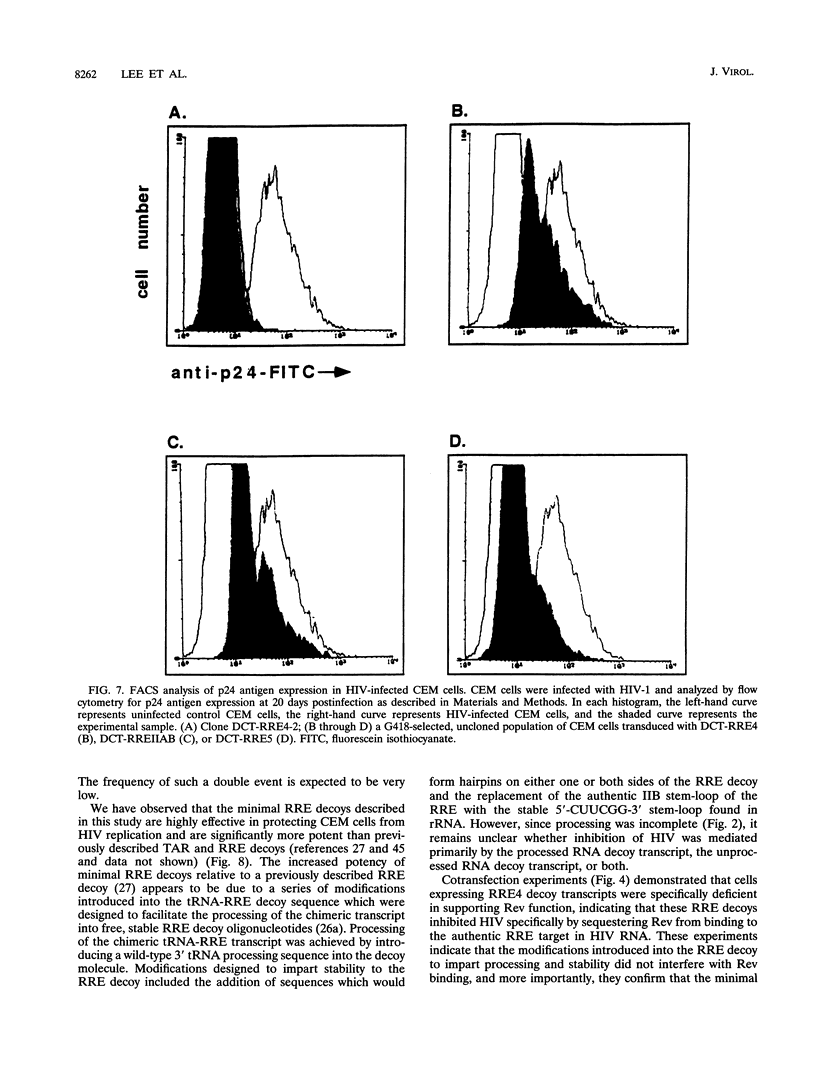
- Rosen C. A., Pavlakis G. N. Tat and Rev: positive regulators of HIV gene expression. AIDS. 1990 Jun;4(6):499–509. [PubMed] [Google Scholar]
- Rounseville M. P., Kumar A. Binding of a host cell nuclear protein to the stem region of human immunodeficiency virus type 1 trans-activation-responsive RNA. J Virol. 1992 Mar;66(3):1688–1694. doi: 10.1128/jvi.66.3.1688-1694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Sanford J. C. Applying the PDR principle to AIDS. J Theor Biol. 1988 Feb 21;130(4):469–480. doi: 10.1016/s0022-5193(88)80211-x. [DOI] [PubMed] [Google Scholar]
- Santos T., Zasloff M. Comparative analysis of human chromosomal segments bearing nonallelic dispersed tRNAimet genes. Cell. 1981 Mar;23(3):699–709. doi: 10.1016/0092-8674(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Sheline C. T., Milocco L. H., Jones K. A. Two distinct nuclear transcription factors recognize loop and bulge residues of the HIV-1 TAR RNA hairpin. Genes Dev. 1991 Dec;5(12B):2508–2520. doi: 10.1101/gad.5.12b.2508. [DOI] [PubMed] [Google Scholar]
- Shukla R. R., Kimmel P. L., Kumar A. Human immunodeficiency virus type 1 Rev-responsive element RNA binds to host cell-specific proteins. J Virol. 1994 Apr;68(4):2224–2229. doi: 10.1128/jvi.68.4.2224-2229.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenger B. A., Gallardo H. F., Ungers G. E., Gilboa E. Analysis of trans-acting response decoy RNA-mediated inhibition of human immunodeficiency virus type 1 transactivation. J Virol. 1991 Dec;65(12):6811–6816. doi: 10.1128/jvi.65.12.6811-6816.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenger B. A., Gallardo H. F., Ungers G. E., Gilboa E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell. 1990 Nov 2;63(3):601–608. doi: 10.1016/0092-8674(90)90455-n. [DOI] [PubMed] [Google Scholar]
- Sullenger B. A., Lee T. C., Smith C. A., Ungers G. E., Gilboa E. Expression of chimeric tRNA-driven antisense transcripts renders NIH 3T3 cells highly resistant to Moloney murine leukemia virus replication. Mol Cell Biol. 1990 Dec;10(12):6512–6523. doi: 10.1128/mcb.10.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiley L. S., Madore S. J., Malim M. H., Cullen B. R. The VP16 transcription activation domain is functional when targeted to a promoter-proximal RNA sequence. Genes Dev. 1992 Nov;6(11):2077–2087. doi: 10.1101/gad.6.11.2077. [DOI] [PubMed] [Google Scholar]
- Tiley L. S., Malim M. H., Tewary H. K., Stockley P. G., Cullen B. R. Identification of a high-affinity RNA-binding site for the human immunodeficiency virus type 1 Rev protein. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):758–762. doi: 10.1073/pnas.89.2.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C., Gauss P., Thermes C., Groebe D. R., Gayle M., Guild N., Stormo G., d'Aubenton-Carafa Y., Uhlenbeck O. C., Tinoco I., Jr CUUCGG hairpins: extraordinarily stable RNA secondary structures associated with various biochemical processes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1364–1368. doi: 10.1073/pnas.85.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav Y. N., Vaishnav M., Wong-Staal F. Identification and characterization of a nuclear factor that specifically binds to the Rev response element (RRE) of human immunodeficiency virus type 1 (HIV-1). New Biol. 1991 Feb;3(2):142–150. [PubMed] [Google Scholar]
- Yamada O., Yu M., Yee J. K., Kraus G., Looney D., Wong-Staal F. Intracellular immunization of human T cells with a hairpin ribozyme against human immunodeficiency virus type 1. Gene Ther. 1994 Jan;1(1):38–45. [PubMed] [Google Scholar]
- Yu M., Poeschla E., Wong-Staal F. Progress towards gene therapy for HIV infection. Gene Ther. 1994 Jan;1(1):13–26. [PubMed] [Google Scholar]