Abstract
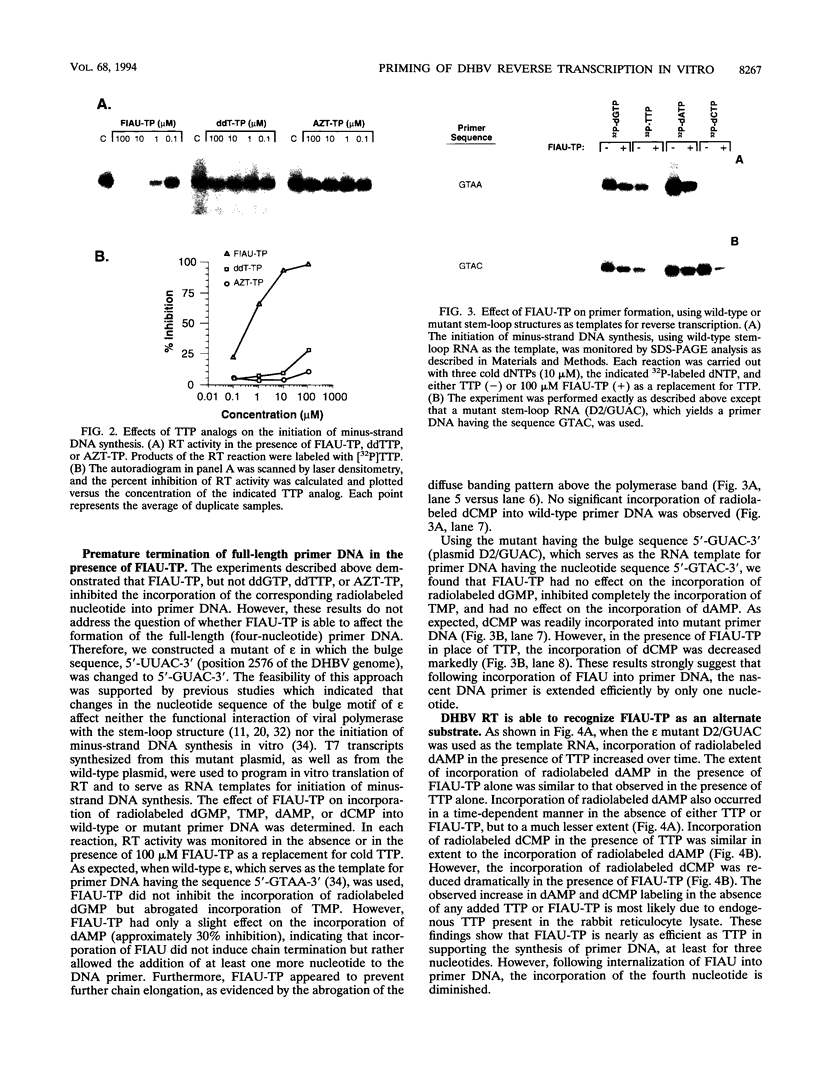
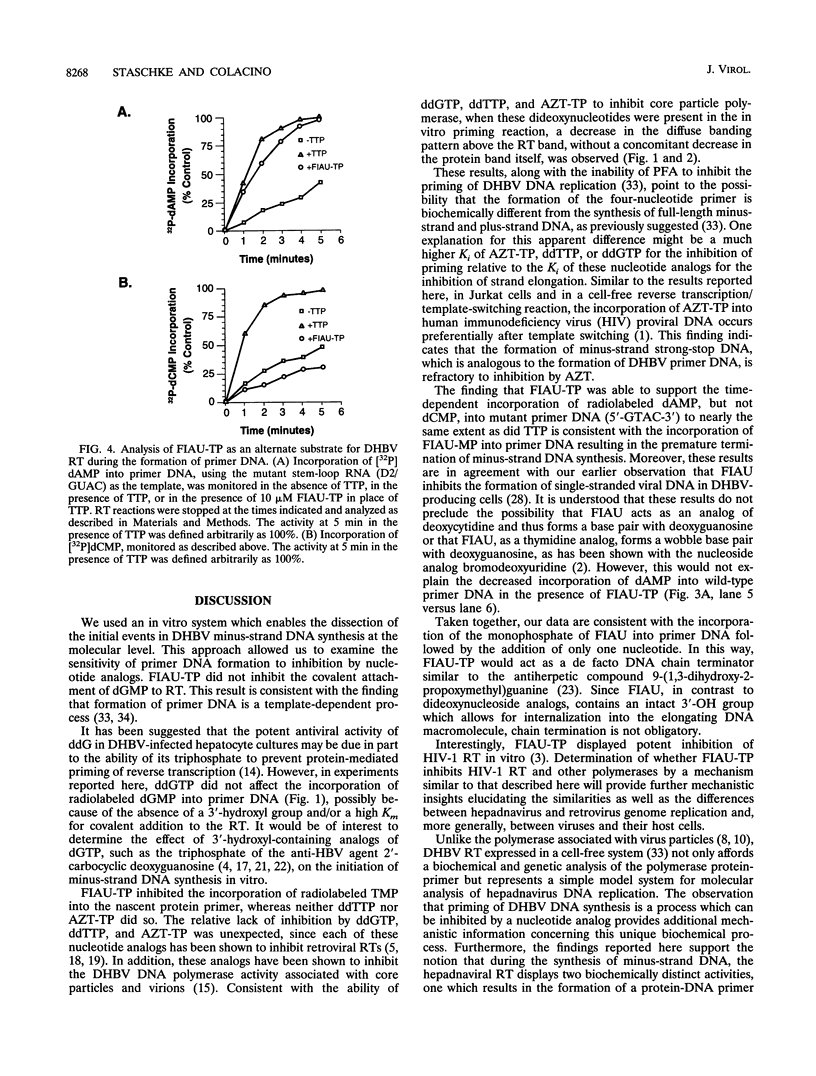
Hepadnaviruses employ a unique mechanism for the initiation of RNA-directed DNA synthesis. Initially, four bases (5'-GTAA-3') are added to a tyrosine residue of the viral polymerase by reverse transcription of a bulge sequence in epsilon, a stem-loop structure which functions as the packaging signal for pregenomic RNA. This protein-DNA complex acts as the primer for minus-strand elongation from the 3' sequence, DR1. To understand this process in greater detail, we investigated whether the protein-mediated priming of viral DNA synthesis is affected by nucleotide analogs. By using cell-free expression of duck hepatitis B virus (DHBV) reverse transcriptase (G.-H. Wang and C. Seeger, Cell 71:663-670, 1992), the 5'-triphosphate of the thymidine analog fialuridine (FIAU) was shown to inhibit the incorporation of radiolabeled TMP into primer DNA in a dose-dependent manner. Inhibition by the 5'-triphosphate of FIAU (FIAU-TP) was nearly complete at a concentration of 10 microM. The dideoxynucleotide analogs ddGTP, ddTTP, and 3'-azidodeoxythymidine triphosphate, known inhibitors of DHBV endogenous DNA polymerase, did not affect substantially the synthesis of primer DNA. Alternate substrate analysis suggested that FIAU is incorporated efficiently into nascent primer DNA as an analog of thymidine. Using site-directed mutagenesis to construct a mutant RNA template yielding a primer with the sequence 5'-GTAC-3', we demonstrated that FIAU-TP inhibited the incorporation of TMP, had no effect on that of dAMP, and decreased markedly the incorporation of dCMP. These results show that the synthesis of full-length DHBV primer DNA is inhibited by FIAU-TP but not by the dideoxynucleotide analogs that we tested. The significance of these findings as they relate to HBV DNA replication is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arts E. J., Wainberg M. A. Preferential incorporation of nucleoside analogs after template switching during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1994 May;38(5):1008–1016. doi: 10.1128/aac.38.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Kneale G., Hunter W. N., Kennard O. Structural characterisation of the bromouracil.guanine base pair mismatch in a Z-DNA fragment. Nucleic Acids Res. 1986 Feb 25;14(4):1801–1809. doi: 10.1093/nar/14.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel I., Saputelli J., Schaffer P., Mason W. S. The carbocyclic analog of 2'-deoxyguanosine induces a prolonged inhibition of duck hepatitis B virus DNA synthesis in primary hepatocyte cultures and in the liver. J Virol. 1994 Feb;68(2):1059–1065. doi: 10.1128/jvi.68.2.1059-1065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Fyfe J. A., St Clair M. H., Weinhold K., Rideout J. L., Freeman G. A., Lehrman S. N., Bolognesi D. P., Broder S., Mitsuya H. Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Hantz O., Allaudeen H. S., Ooka T., De Clercq E., Trepo C. Inhibition of human and woodchuck hepatitis virus DNA polymerase by the triphosphates of acyclovir, 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-5-iodocytosine and E-5-(2-bromovinyl)-2'-deoxyuridine. Antiviral Res. 1984 Aug;4(4):187–199. doi: 10.1016/0166-3542(84)90017-2. [DOI] [PubMed] [Google Scholar]
- Hirschman S. Z., Vernace S. J., Schaffner F. D.N.A. polymerase in preparations containing Australia antigen. Lancet. 1971 May 29;1(7709):1099–1103. doi: 10.1016/s0140-6736(71)91839-3. [DOI] [PubMed] [Google Scholar]
- Howe A. Y., Elliott J. F., Tyrrell D. L. Duck hepatitis B virus polymerase produced by in vitro transcription and translation possesses DNA polymerase and reverse transcriptase activities. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1170–1176. doi: 10.1016/0006-291x(92)92327-t. [DOI] [PubMed] [Google Scholar]
- Kaplan P. M., Greenman R. L., Gerin J. L., Purcell R. H., Robinson W. S. DNA polymerase associated with human hepatitis B antigen. J Virol. 1973 Nov;12(5):995–1005. doi: 10.1128/jvi.12.5.995-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus T., Nassal M. The encapsidation signal on the hepatitis B virus RNA pregenome forms a stem-loop structure that is critical for its function. Nucleic Acids Res. 1993 Aug 25;21(17):3967–3975. doi: 10.1093/nar/21.17.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Gerin J. L. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antiviral Res. 1992 Jul 1;19(1):55–70. doi: 10.1016/0166-3542(92)90056-b. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee B., Luo W. X., Suzuki S., Robins M. J., Tyrrell D. L. In vitro and in vivo comparison of the abilities of purine and pyrimidine 2',3'-dideoxynucleosides to inhibit duck hepadnavirus. Antimicrob Agents Chemother. 1989 Mar;33(3):336–339. doi: 10.1128/aac.33.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfgren B., Nordenfelt E., Oberg B. Inhibition of RNA- and DNA-dependent duck hepatitis B virus DNA polymerase activity by nucleoside and pyrophosphate analogs. Antiviral Res. 1989 Dec;12(5-6):301–310. doi: 10.1016/0166-3542(89)90057-0. [DOI] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Cullen J., Saputelli J., Wu T. T., Liu C., London W. T., Lustbader E., Schaffer P., O'Connell A. P., Fourel I. Characterization of the antiviral effects of 2' carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology. 1994 Feb;19(2):398–411. [PubMed] [Google Scholar]
- Ono K., Ogasawara M., Iwata Y., Nakane H., Fujii T., Sawai K., Saneyoshi M. Inhibition of reverse transcriptase activity by 2',3'-dideoxythymidine 5'-triphosphate and its derivatives modified on the 3' position. Biochem Biophys Res Commun. 1986 Oct 30;140(2):498–507. doi: 10.1016/0006-291x(86)90760-6. [DOI] [PubMed] [Google Scholar]
- Parker W. B., White E. L., Shaddix S. C., Ross L. J., Buckheit R. W., Jr, Germany J. M., Secrist J. A., 3rd, Vince R., Shannon W. M. Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase and human DNA polymerases alpha, beta, and gamma by the 5'-triphosphates of carbovir, 3'-azido-3'-deoxythymidine, 2',3'-dideoxyguanosine and 3'-deoxythymidine. A novel RNA template for the evaluation of antiretroviral drugs. J Biol Chem. 1991 Jan 25;266(3):1754–1762. [PubMed] [Google Scholar]
- Pollack J. R., Ganem D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993 Jun;67(6):3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. M., Banerjee R., Acs G. Inhibition of the replication of hepatitis B virus by the carbocyclic analogue of 2'-deoxyguanosine. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8541–8544. doi: 10.1073/pnas.86.21.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. M., Banerjee R., Jeffrey A. M., Acs G. The mechanism of inhibition of hepatitis B virus replication by the carbocyclic analog of 2'-deoxyguanosine. Hepatology. 1992 Jul;16(1):8–12. doi: 10.1002/hep.1840160103. [DOI] [PubMed] [Google Scholar]
- Reardon J. E. Herpes simplex virus type 1 and human DNA polymerase interactions with 2'-deoxyguanosine 5'-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. J Biol Chem. 1989 Nov 15;264(32):19039–19044. [PubMed] [Google Scholar]
- Seeger C., Maragos J. Identification and characterization of the woodchuck hepatitis virus origin of DNA replication. J Virol. 1990 Jan;64(1):16–23. doi: 10.1128/jvi.64.1.16-23.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Maragos J. Identification of a signal necessary for initiation of reverse transcription of the hepadnavirus genome. J Virol. 1991 Oct;65(10):5190–5195. doi: 10.1128/jvi.65.10.5190-5195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staschke K. A., Colacino J. M., Mabry T. E., Jones C. D. The in vitro anti-hepatitis B virus activity of FIAU [1-(2'-deoxy-2'-fluoro-1-beta-D-arabinofuranosyl-5-iodo)uracil] is selective, reversible, and determined, at least in part, by the host cell. Antiviral Res. 1994 Jan;23(1):45–61. doi: 10.1016/0166-3542(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Tavis J. E., Ganem D. Expression of functional hepatitis B virus polymerase in yeast reveals it to be the sole viral protein required for correct initiation of reverse transcription. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4107–4111. doi: 10.1073/pnas.90.9.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Tong S. P., Li J. S., Vitvitski L., Trépo C. Replication capacities of natural and artificial precore stop codon mutants of hepatitis B virus: relevance of pregenome encapsidation signal. Virology. 1992 Nov;191(1):237–245. doi: 10.1016/0042-6822(92)90185-r. [DOI] [PubMed] [Google Scholar]
- Wang G. H., Seeger C. Novel mechanism for reverse transcription in hepatitis B viruses. J Virol. 1993 Nov;67(11):6507–6512. doi: 10.1128/jvi.67.11.6507-6512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. H., Seeger C. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell. 1992 Nov 13;71(4):663–670. doi: 10.1016/0092-8674(92)90599-8. [DOI] [PubMed] [Google Scholar]
- Weber M., Bronsema V., Bartos H., Bosserhoff A., Bartenschlager R., Schaller H. Hepadnavirus P protein utilizes a tyrosine residue in the TP domain to prime reverse transcription. J Virol. 1994 May;68(5):2994–2999. doi: 10.1128/jvi.68.5.2994-2999.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulim F., Seeger C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J Virol. 1994 Jan;68(1):6–13. doi: 10.1128/jvi.68.1.6-13.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]