Abstract
Inhibitors of the protease of HIV-1 have been used successfully for the treatment of HIV-1-infected patients and AIDS disease. We tested whether these protease inhibitory drugs exerted effects in addition to their antiviral activity. Here, we show in mice infected with lymphocytic choriomeningitis virus and treated with the HIV-1 protease inhibitor ritonavir a marked inhibition of antiviral cytotoxic T lymphocyte (CTL) activity and impaired major histocompatibility complex class I-restricted epitope presentation in the absence of direct effects on lymphocytic choriomeningitis virus replication. A potential molecular target was found: ritonavir selectively inhibited the chymotrypsin-like activity of the 20S proteasome. In view of the possible role of T cell-mediated immunopathology in AIDS pathogenesis, the two mechanisms of action (i.e., reduction of HIV replication and impairment of CTL responses) may complement each other beneficially. Thus, the surprising ability of ritonavir to block the presentation of antigen to CTLs may possibly contribute to therapy of HIV infections but potentially also to the therapy of virally induced immunopathology, autoimmune diseases, and transplantation reactions.
A three-drug regimen containing a protease inhibitor and two nucleoside analogs is the most effective treatment available for HIV-1 infection to date (1–3). However, there are many unanswered questions regarding duration of therapy, exact mechanism of actions, prospects of long-term therapy, emergence of resistance, and long-term toxicity. The extent of immunological recovery with treatment has been debated. Unexpectedly, in some patients treated with protease inhibitors, virus titers were not drastically altered but CD4 T cells increased remarkably (4); in a few other patients, transient reactivation of other persistent viruses during treatment has been observed (5–7). Cytotoxic T lymphocytes (CTLs) play an important role in early defense, especially against several noncytopathic infections and against HIV-1. HIV-1 protease inhibitors may have other effects in addition to interfering with HIV replication. Here, we investigated the influence of the HIV-1 protease inhibitor ritonavir on immune responses in general and on in vivo CTL activity in particular. As a model, we used the infection of mice with lymphocytic choriomeningitis virus (LCMV), a noncytopathic arenavirus infection in which CTLs are responsible both for the initial viral control and for virus-induced immunopathological disease (8, 9). Our results indicate that ritonavir is a modulator of proteasome activity and major histocompatibility complex (MHC) class I-restricted presentation of several LCMV and MART-1 epitopes in vivo and in vitro and may help to explain some of the observations made in AIDS patients undergoing highly active antiretroviral therapy.
MATERIALS AND METHODS
Mice.
C57BL/6 and BALB/c mice were purchased from Institut für Zuchthygiene (Tierspital Zürich, Switzerland) and held in a specific pathogen-free mouse housing facility according to Swiss federal animal regulations.
Measurement of Footpad Swelling Reactions.
Mice were infected in the footpad with 30 μl of either 104 plaque-forming units (pfu)/ml LCMV-WE (F. Lehmann-Grube, Pette-Institute, Germany), 104 pfu/ml LCMV-Armstrong (M. Buchmeier, Scripps Clinic, La Jolla, CA), or 10 pfu/ml LCMV-Docile (C. Pfau, Rensselaer Polytechnic Institute, Troy, NY). Treatment was with ritonavir dissolved in 10% alcohol and 90% PBS either i.p. or orally by using an intragastric sonde; control mice were treated identically with 10% alcohol/PBS. Footpad measurements were taken daily (10). Viruses were titrated as detailed elsewhere (11).
Cytolytic Assays.
Mice were infected by an i.v. injection of 200 pfu of LCMV-WE; spleen cell suspensions were prepared 8 days later. Lytic activities were determined in a standard 4-h 51Cr release assay (12). The LCMV–glycoprotein (GP)-transfected cell line MC57-GP has been described previously (13).
Flow Cytometry.
MC57 mouse fibrosarcoma cells were treated with 7 μM of ritonavir in MEM with 10% fetal calf serum and infected for 48 h with LCMV-WE at a multiplicity of infection of 0.04. Surface LCMV–GP staining was performed by using mAb KL-25 and intracellular nucleoprotein (NP) staining by (0.1%) saponin permeabilization of cells followed by KL-53 staining (14). The cells were washed and stained with goat anti-rat IgG-fluorescein isothiocyanate (Tago) and analyzed on an Epics Profile flow cytometer (Coulter). MHC class I cell-surface staining of M113 and EL4 cells was performed by using W6/32 and B22/249 mAbs, respectively.
Immunoprecipitation.
For analyses of MHC class I maturation and expression, M113 and EL4 cells were cultured overnight in 5 μg/ml ritonavir, preincubated in methionine and cysteine-free medium for 1 h, pulsed with [35S]methionine and [35S]cysteine, washed in cold PBS, and lysed as shown. After lysis in 1% Triton X-100 PBS and preclearing, lysates were immunoprecipitated with mAbs W6/32 (M113 lysates) or B22/249 (EL4 lysates). Endo-H treatment was performed overnight according to the manufacturer’s instructions (Boehringer Mannheim). Samples were heated for 3 min at 100°C with 2-mercaptoethanol before SDS/PAGE.
T Cell Stimulation Assays.
M113 melanoma cells were cultured for 24 h in RPMI 1640 medium supplemented with 10% fetal calf serum and various concentrations of ritonavir. Cells then were resuspended, fixed in PBS containing 1% paraformaldehyde for 5 min at 37°C, and washed extensively. A total of 2 × 104 M77-84 T cells were added to 105 stimulating, fixed M113 cells in 200 μl of medium. Tumor necrosis factor α production in the supernatant of 24-h cultures was measured by taking advantage of the tumor necrosis factor α cytotoxic effect on WEHI cells; it was expressed as the percentage of maximal cytotoxicity obtained with untreated M113 cells in a standard colorimetric assay. M77-84 proliferation was measured by adding [3H]thymidine (1 μCi/well; 1 Ci = 37 GBq) to the medium during the last 18 h of a 48-h culture.
RESULTS
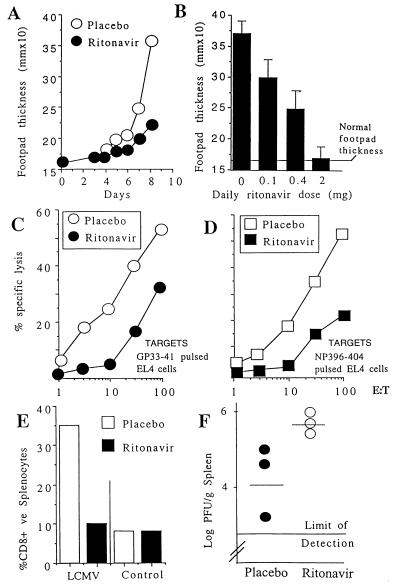
As an initial experiment to test whether the HIV-1 protease inhibitor ritonavir would affect the CTL response in the mouse, we examined the swelling of footpads of C57BL/6 mice that had been injected 7–8 days before with LCMV-WE (300 pfu). The swelling of footpads caused by local LCMV infection is a direct in vivo measure of CTL activity (10). As shown in Fig. 1 A and B, footpad swelling reactions were markedly inhibited by ritonavir treatment in a dose-dependent manner. Similar results were obtained with different virus strains (LCMV-Docile and -Armstrong), different mouse strains, and both parenteral and oral routes of administration of the drug. It is unlikely that a reduction in the CTL response against LCMV is caused by a direct inhibitory effect on LCMV replication because LCMV does not encode protease and treatment of MC57 fibroblast cells with ≤10 μM of ritonavir did not affect the production of LCMV-WE in the supernatant at 48 h postinfection with a multiplicity of infection of 0.04 (data not shown).
Figure 1.
Effect of ritonavir on CTL activity in vivo. (A and B) Inhibition of footpad swelling after LCMV infection: (A) C57BL/6 mice were infected in the footpad on day 0 (300 pfu of LCMV-WE), and footpad swelling was measured over time. Ritonavir (1.25 mg/mouse/day) or placebo control (same volume of PBS and 10% alcohol) were administered i.p. from day 0. In this and subsequent experiments, readings are from two footpads from 2–3 mice per group and are representative of 3–4 separate experiments. (B) Footpad swelling induced by LCMV infection was measured on day 8 in mice treated with varying doses of ritonavir. (C and D) Inhibition of ex vivo CTL activity. Ritonavir-treated (1.25 mg/mouse/day) or placebo-treated C57BL/6 mice were infected with 200 pfu of LCMV-WE i.v. At 8 days postchallenge, splenocytes were tested for lysis of EL4 cells (H-2b) prepulsed with 500 nM peptide GP33–41 (KAVYNFATC; C) or NP396–407 (FQPQNGQFI; D). E/T, effector to target ratio. (E) Inhibition of CD8+ T cell expansion in vivo. Mice were infected with 200 pfu of LCMV-WE i.v. and treated with ritonavir or placebo as above. Splenocytes were stained with fluorescein isothiocyanate-conjugated anti-CD8 (PharMingen), and the percentage of positive cells was determined by flow cytometry. (F) Effect of ritonavir on LCMV viral load. Mice were infected with 200 pfu of LCMV i.v. and treated with ritonavir or placebo as in C. Spleen virus titer was determined on day 8.
Treatment with ritonavir also inhibited the CTL response to systemic LCMV infection. First, specific lysis of target cells expressing peptides derived from LCMV–GP (GP33–41) or LCMV–NP (NP396–404) by splenocytes taken directly from LCMV-primed mice were reduced when mice were treated with ritonavir during the 8 days of infection (Fig. 1 C and D). Second, there was inhibition of the normally observed LCMV-induced expansion of CD8+ T cells to the virus in vitro (Fig. 1E). Third, the reduced lysis of target cells was accompanied by impaired virus clearance in vivo (Fig. 1F), a result that confirms that this drug acts on the immune response rather than through an antiviral effect on LCMV. In contrast, ritonavir did not affect the percentages of CD4 and CD8 or total splenocyte counts in uninfected mice, nor did it impair the ability of mice to mount antiviral T-independent IgM and T-dependent IgG antibody responses against LCMV–NP or –GP as measured by ELISA at 4 and 8 wk postinfection (15) or against vesicular stomatitis virus at 4, 6, and 12 days postinfection (16) (data not shown). The effects of ritonavir on CTL responses in LCMV-WE-infected animals were partial and reversible. Immunological memory was generated under ritonavir treatment; mice treated with ritonavir and infected i.v. with 2 × 106 pfu of LCMV-WE or -Docile were able to mount CTL responses in vivo and clear the virus from the spleen within 4 days after an i.v. rechallenge with 2 × 106 pfu at 2 wk after the primary challenge (data not shown).
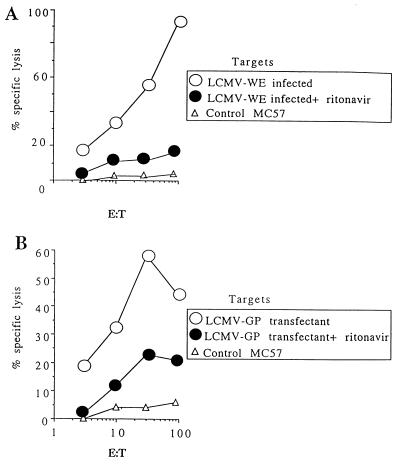
The results so far indicated that ritonavir might impair mechanisms of antigen (Ag) presentation to CD8+ T cells. In fact, incubation of LCMV-infected MC57 fibroblast cells with ritonavir inhibited lysis by CTLs specific for GP33–41 (Fig. 2A) and NP396–404 (data not shown). This treatment had no effect on the production of LCMV in the supernatant, on cell surface expression of LCMV–GP, or on intracellular expression of LCMV–NP in infected cells according to flow cytometric analysis. A similar inhibitory effect also was seen with ritonavir-treated MC57 cells transfected with LCMV–GP (13) (Fig. 2B), but there was no inhibition of lysis of uninfected MC57 or EL4 cells pulsed with GP33–41 peptide (data not shown).
Figure 2.
(A) Effect of ritonavir on MHC class I-restricted presentation in vitro as determined by CTL lysis of LCMV-WE-infected targets. (B) GP33–41-specific CTLs were prepared by secondary restimulation in vitro from spleens of LCMV-primed mice. LCMV-WE infection of MC57 fibroblasts (H-2b) was performed at a multiplicity of infection of 0.04, ritonavir was added at a concentration of 5 μg/ml, and the cells were cultured for 36 h. Effect of ritonavir on lysis of LCMV–GP-transfected targets. Targets were LCMV–GP-transfected MC57 cells as described in ref. 13. Effectors were splenocytes from C57BL/6 mice at 8 days after 200 pfu LCMV-WE infection. Targets were treated with ritonavir or left untreated as in A.
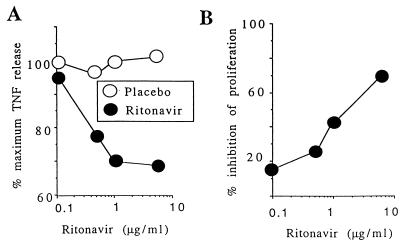
We examined whether ritonavir also would modulate Ag presentation to human CTL clones by testing proliferation and tumor necrosis factor α release by MART-1-specific M77-84 T cells (17, 18) after stimulation by M113 melanoma cells grown in the presence and absence of ritonavir (Fig. 3). Inhibition was dose-dependent and effective in the range of therapeutic doses, because it was also seen when Ag-expressing cells were grown in serum from an HIV-negative volunteer after ingestion of a single oral dose of 500 mg of ritonavir. Up to a 70% inhibition of proliferation was observed with serum taken 3 h after ingestion of ritonavir, corresponding to peak rather than steady-state plasma concentrations of ritonavir (3). However, ritonavir modulated CTL-mediated cytolysis of melanoma cells only marginally under the conditions used (data not shown).
Figure 3.
Effect of ritonavir on MHC class I presentation to human T cell clones. (A) M113 melanoma cells were cultured for 24 h with various concentrations of ritonavir, resuspended, and fixed in paraformaldehyde. The same concentrations of indinavir were used as a control (placebo). Tumor necrosis factor α production by HLA-A2-restricted T cells specific for the MART-1 epitope 26–35 (M77-84; ref. 18) was measured in the supernatant and is expressed as the percentage of maximal release obtained with untreated stimulator M113 cells. (B) M113 cells were cultured for 24 h in ritonavir (0.1–5 μg/ml) and then used as stimulators in a proliferation assay with M77-84 T cells as responders. Proliferation was measured over 48 h with thymidine added at 1 μCi/well for the last 18 h.
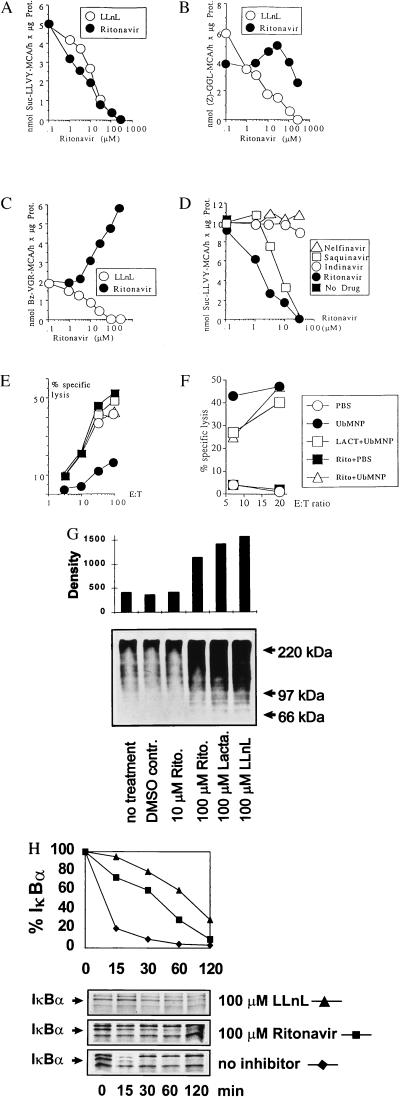
The proteasome is a protein degradation system that is centrally involved in the production of T cell epitopes (19). Therefore, we examined whether the HIV-1 protease inhibitor ritonavir would have an effect on peptide hydrolysis by isolated murine or human 20S proteasomes. Ritonavir inhibited the chymotrypsin-like activity of the proteasome, which is commonly measured by hydrolysis of the fluorogenic tyrosine substrate Suc-LLVY-MCA, as effectively as the proteasome inhibitor N-acetyl-leucyl-leucyl-norleucinal (LLnL) (Fig. 4A). However, the hydrolysis of the leucine substrate Z-GGL-MCA was barely affected, and cleavage of the arginine substrate Bz-VGR-MCA was consistently enhanced (Fig. 4 B and C). This selective inhibition by ritonavir is in contrast to peptide aldehyde inhibition by LLnL, which is known to covalently block all active sites of 20S proteasomes (20, 21) and which inhibited the hydrolysis of the three tested fluorogenic substrates.
Figure 4.
Chymotrypsin-like activity of the proteasome is inhibited by ritonavir. Hydrolysis of fluorogenic substrates (A) 100 μM Suc-LLVY-MCA, (B) 100 μM Z-GGL-MCA, and (C) 400 μM Bz-VGR-MCA by isolated 20S proteasomes from murine B8 fibroblast cells were plotted vs. indicated concentrations of ritonavir and the protea- some inhibitor LLnL. The results of 1-h digests with 500 ng of 20S proteasome in a final volume 100 μl are shown (29). Values were in a linear range of detection and are the means of triplicates with SE of <3% for all data points. (D) Comparison of four HIV-1 protease inhibitors. Hydrolysis of Suc-LLVY-MCA by murine 20S proteasomes was tested as described in B under identical conditions, except that a different batch of purified proteasomes was used. (E) Comparison of four protease inhibitors: effect on CTL lysis ex vivo. C57BL/6 mice (2–3 animals per group) that had been primed previously with LCMV-WE were rechallenged (2 × 106 pfu of LCMV-WE i.v.) and treated with either placebo or 4 mg of ritonavir, saquinavir, indinavir, or nelfinavir orally once daily for 4 days. Splenocytes then were tested directly ex vivo for lysis of NP396–404-pulsed EL4 targets. Lysis of unpulsed targets was <3%. (F) Ritonavir does not affect the presentation of the proteasome-independent influenza NP epitope 50–57 (30). LKd cells were infected with a vaccinia recombinant expressing a fusion protein of ubiquitin and influenza NP (UbMNP). Ritonavir treatment was for 36 h at 10 μM and lactacystin treatment was for 30 min at 100 μM before cells were infected with 10 pfu/cell of recombinant vaccinia virus. Labeling with 100 μCi 51Cr occurred for 90 min during infection; next, recombinant proteins were allowed to be expressed for 4 h at 37°C before usage as target cells in a 4-h cytolytic assay by using H-2Kk/NP epitope 50–57-specific polyclonal CTLs as effectors. (G) Accumulation of ubiquitin conjugates in ritonavir-treated cells. B8 murine fibroblast cells were treated for 3 h as indicated, and cellular lysates were analyzed by Western blots probed with an ubiquitin conjugate-reactive antiserum (Dako). The densitometric evaluation of enhanced chemiluminescence-exposed films was normalized to the amount of total protein on Ponceau-stained blots. (H) Inhibition of IκBα degradation in ritonavir-treated cells. NFS5.3 murine B cells were starved for 30 min and metabolically labeled with [35S]-methionine/[35S]-cysteine for 1 h in the presence or absence of inhibitors. Cells were treated with a final concentration of 45 μg/ml lipopolysaccharide before chasing for indicated time periods. Immunoprecipitation was performed with an IκBα-specific polyclonal antibody (Santa Cruz Biotechnology). IkBα bands were quantified on a BAS1500 Imager (Fuji) and normalized to proteasome subunit MC3 precipitated from the same lysate.
To test the effect of other commercially available HIV-1 protease inhibitors, the hydrolysis of Suc-LLVY-MCA by murine 20S proteasomes was measured in the presence of increasing concentrations of ritonavir, saquinavir, indinavir, and nelfinavir (Fig. 4D). Interestingly, apart from ritonavir, only saquinavir inhibited chymotrypsin-like activity, although much less efficiently, whereas no inhibition at all was observed with indinavir and nelfinavir (Fig. 4D). Consistent with this observation, footpad swelling after LCMV injection and direct ex vivo lysis after systemic infection (Fig. 4E) were not inhibited by indinavir or nelfinavir. Some inhibition of footpad swelling could be observed with saquinavir (≤73% inhibition on day 7 after daily treatment with 4 mg orally), but this inhibition was less consistent and sustained than that seen with ritonavir, possibly because of the poor bioavailability of this compound (data not shown). Therefore, an apparently specific and selective proteasome inhibition by ritonavir and, to a lesser extent, saquinavir may account for the inhibitory effects on Ag presentation observed in vivo. Recently, it was reported that an H-2Kk-restricted epitope from influenza NP50–57 was lactacystin insensitive and therefore proteasome independent. If the in vivo effects on Ag presentation were caused by proteasome inhibition, ritonavir should not affect the generation of this proteasome-independent epitope; as shown in Fig. 4F, this was indeed the case.
A hallmark of proteasome inhibition is the intracellular accumulation of polyubiquitinated substrate proteins. A marked increase in ubiquitin conjugates was detected when murine B cells or fibroblasts were treated for 3 h with either 100 μM of ritonavir or the characterized proteasome inhibitors lactacystin and LLnL (Fig. 4G). Moreover, the lipopolysaccharide-induced degradation of the well-defined proteasome substrate IκBα was inhibited at 100 μM of ritonavir or LLnL (Fig. 4H). Taken together, these data indicate that ritonavir is an inhibitor of the proteasome in intact cells, and that modulation of proteasome activity may account for the inhibition of Ag presentation by ritonavir.
DISCUSSION
HIV-1 protease inhibitors are masterpieces of rational drug design and offer great promises for AIDS therapy. Recent clinical data may suggest that these inhibitors exert additional effects on the immune system. Our studies suggest that the HIV-1 protease inhibitor ritonavir may adversely affect the CTL response to LCMV in vivo via interference with MHC class I-restricted presentation of LCMV T cell epitopes. While looking for a molecular target, we found that ritonavir appeared to inhibit the chymotrypsin-like activity of isolated 20S proteasomes in vitro, and that cellular functions of the proteasome such as the degradation of ubiquitin conjugates and IκB are reduced at higher concentrations. The dose required for marked inhibition of Ag presentation in our systems (5 μM) is close to peak serum levels in ritonavir-treated patients (3) and is the concentration required for a 50% inhibition of proteasomal chymotrypsin-like activity in vitro. However, a block in proteasomal housekeeping functions such as ubiquitin conjugate and IκB degradation was not seen at 5 μM but required ≈50–100 μM of the drug. Thus, it appears that Ag presentation is impaired by ritonavir at concentrations where essential functions of the proteasome are not yet blocked. Moreover, a significant reduction of MHC class I maturation and cell surface expression was only seen at 100 μM but not at 5 μM (data not shown). A possible explanation for this finding is a selective inhibition of proteasome activities. This may well account for a marked decrease in the production of the examined LCMV and MART-1 epitopes, whereas the bulk supply of MHC class I ligands is still maintained. However, because conclusive evidence for selective proteasome inhibition is difficult to obtain from cellular experiments, we cannot rule out the involvement of additional and thus far unidentified target proteins in the ritonavir-mediated inhibition of Ag presentation.
Cytotoxic lymphocytes contribute to the initial and long-term control of viral replication through the lysis of infected cells and cytokine production; however, they may also cause immunopathology, as they do in LCMV infections (8, 9, 22, 23). Because AIDS may partially result from immunopathological damage by anti-HIV CTLs, the direct effects of ritonavir on class I-restricted Ag presentation may reduce the immunopathological destruction of Ag-presenting cells, CD4+ lymphocytes, and other HIV-infected cells. Interestingly, reductions in HIV Ag-specific CTL responses may perhaps explain why those patients whose virus is not controlled by ritonavir through the emergence of drug resistance still may show elevated CD4 counts and clinical benefits from ritonavir therapy (4, 24).
Alternatively, reduction in immune surveillance by CTLs through the action of some protease inhibitors may enhance transient infections by hepatitis B virus and hepatitis C virus or facilitate tumor growth in rare patients (25, 26). Although such incidences seem to be exceptional, careful prospective studies seem warranted. The possible effects of HIV-1 protease inhibitors on immunosurveillance probably will depend on the particular role of proteasome-dependent as opposed to proteasome-independent CTL epitope presentation as well as on the relative contributions of the CTL response as opposed to the recovery of CD4+ lymphocytes and antibody production (27–28) in immune surveillance. It will be interesting to compare protocols that include drugs with proteasome-inhibitory capacity (ritonavir/saquinavir) with those apparently lacking this activity (indinavir/nelfinavir) in terms of antiviral effects and CTL numbers or function over time. Finally, the surprising ability of ritonavir to modulate the presentation of Ags to CTLs may perhaps be exploited further for the treatment of autoimmune disease, chronic immunopathologies, or disease caused by transplantation reactions.
Acknowledgments
We thank Frances Yen, Anne-Pascale Satie, Pascale Turmel, Christian Michelet, Edit Horvath, Alana Althage, Constantino Lopez-Macia, Adrian Ciurea, Andrew McPherson, Richard Cone, and Marie-Alix Peyrat for excellent support, discussion, and technical assistance. This work was supported by Institut Nationale de la Santé et de la Recherche Médicale, Sidaction, Agence Nationale de Recherches sur le SIDA, Ligue Nationale Française Contre le Cancer, the Wellcome Trust, the Medical Research Council of Great Britain, the Roche Research Foundation, the Novartis Foundation, Swiss National Science Foundation Grants 31-50900.97 and 32-53674.98, and the Kanton of Zurich.
ABBREVIATIONS
- LCMV
lymphocytic choriomeningitis virus
- CTL
cytotoxic T lymphocyte
- MHC
major histocompatibility complex
- pfu
plaque-forming unit
- GP
glycoprotein
- NP
nucleoprotein
- LLnL
N-acetyl-leucyl-leucyl-norleucinal
- Ag
antigen
References
- 1. Richman D D. Science. 1996;272:1886–1888. doi: 10.1126/science.272.5270.1886. [DOI] [PubMed] [Google Scholar]
- 2.Danner S A, Carr A, Leonard J M, Lehman L M, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V, et al. N Engl J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 3.Kempf D J, Marsh K C, Denissen J F, McDonald E, Vasavanonda S, Flentge C A, Green B E, Fino L, Park C H, Kong X, et al. Proc Natl Acad Sci USA. 1995;92:2484–2488. doi: 10.1073/pnas.92.7.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrin L, Telenti A. Science. 1998;280:1871–1873. doi: 10.1126/science.280.5371.1871. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson M A, Zegans M, Pavan P R, O’Donnell J J, Sattler F, Rao N, Owens S, Pollard R. Lancet. 1997;349:1443–1445. doi: 10.1016/S0140-6736(96)11431-8. [DOI] [PubMed] [Google Scholar]
- 6.Carr A, Cooper D A. Lancet. 1997;349:995–996. doi: 10.1016/S0140-6736(05)62892-9. [DOI] [PubMed] [Google Scholar]
- 7.Rutschmann O T, Negro F, Hirschel B, Hadengue A, Anwar D, Perrin L H. J Infect Dis. 1998;177:783–785. doi: 10.1086/517808. [DOI] [PubMed] [Google Scholar]
- 8.Zinkernagel R M, Doherty P C. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- 9.Buchmeier M J, Welsh R M, Dutko F J, Oldstone M B A. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 10.Zinkernagel R M, Leist T, Hengartner H, Althage A. J Exp Med. 1985;162:2125–2141. doi: 10.1084/jem.162.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel R M. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 12.Pircher H, Baenziger J, Schilham M, Sado T, Kamisaku H, Hengartner H, Zinkernagel R M. Eur J Immunol. 1987;17:159–166. doi: 10.1002/eji.1830170202. [DOI] [PubMed] [Google Scholar]
- 13.Kündig T M, Bachmann M F, DiPaolo C, Simard J J, Battegay M, Lother H, Gessner A, Kuhlcke K, Ohashi P S, Hengartner H, et al. Science. 1995;268:1343–1347. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 14.Zeller W, Bruns M, Lehmann-Grube F. Virology. 1988;162:90–97. doi: 10.1016/0042-6822(88)90397-2. [DOI] [PubMed] [Google Scholar]
- 15.Planz O, Seiler P, Hengartner H, Zinkernagel R M. Nature (London) 1996;382:726–729. doi: 10.1038/382726a0. [DOI] [PubMed] [Google Scholar]
- 16.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, Aguet M, Hengartner H, Zinkernagel R M. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins P F, Rivoltini L, Yannelli J R, Appela E, Rosenberg S A. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gervois N, Guilloux Y, Diez E, Jotereau F. J Exp Med. 1996;183:2403–2407. doi: 10.1084/jem.183.5.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groettrup M, Soza A, Kuckelkorn U, Kloetzel P M. Immunol Today. 1996;17:429–435. doi: 10.1016/0167-5699(96)10051-7. [DOI] [PubMed] [Google Scholar]
- 20.Groll M, Ditzel L, Löwe J, Stock D, Bochtler M, Bartunik H D, Huber R. Nature (London) 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 21.Cerundolo V, Benham A, Braud V, Mukherjee S, Gould K, Macino B, Neefjes J, Townsend A. Eur J Immunol. 1997;27:336–341. doi: 10.1002/eji.1830270148. [DOI] [PubMed] [Google Scholar]
- 22.Heinkelein M, Euler-Konig I, Klinker H, Ruckle-Lanz H, Jassoy C. J Infect Dis. 1996;174:209–213. doi: 10.1093/infdis/174.1.209. [DOI] [PubMed] [Google Scholar]
- 23.Klenerman P, Zinkernagel R M. Immunol Rev. 1997;159:5–16. doi: 10.1111/j.1600-065x.1997.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 24.Cameron D W, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S, Maurath C, Sun E, Henry D, Rode R, Potthoff A, Leonard J. Lancet. 1998;351:543–549. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 25.Kersten M J, Klein M R, Holwerda A M, Miedema F, van Oers M H. J Clin Invest. 1997;99:1525–1533. doi: 10.1172/JCI119315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gershengorn M C, Mesri E A. Nature (London) 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 28.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 29.Groettrup M, Ruppert T, Kuehn L, Seeger M, Standera S, Koszinowski U, Kloetzel P M. J Biol Chem. 1995;270:23808–23815. doi: 10.1074/jbc.270.40.23808. [DOI] [PubMed] [Google Scholar]
- 30.Anton L C, Snyder H L, Bennink J R, Vinitsky A, Orlowski M, Porgador A, Yewdell J W. J Immunol. 1998;160:4859–4868. [PubMed] [Google Scholar]