Abstract
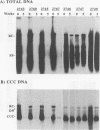
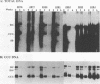
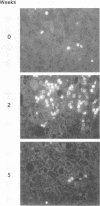
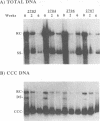
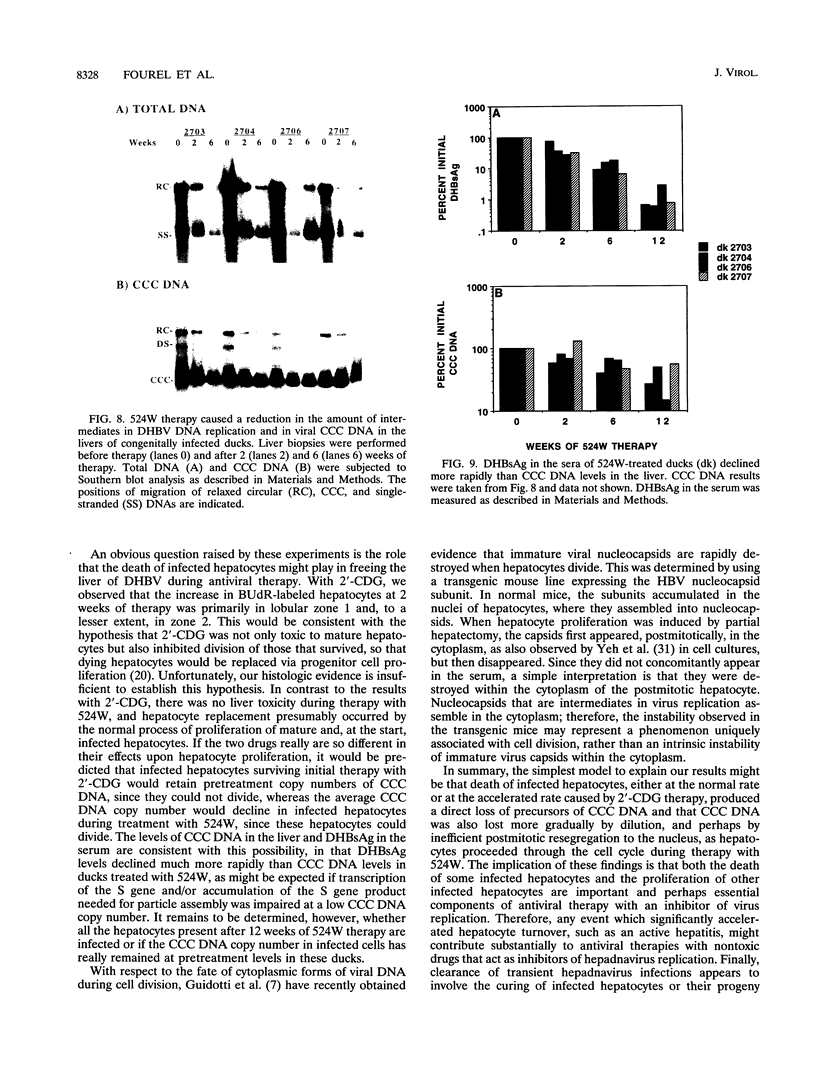
Duck hepatitis B virus (DHBV) DNA synthesis in congenitally infected ducks is inhibited by 2'-deoxycarbocyclic guanosine (2'-CDG). Three months of therapy reduces the number of infected hepatocytes at least 10-fold (W.S. Mason, J. Cullen, J. Saputelli, T.-T. Wu, C. Liu, W.T. London, E. Lustbader, P. Schaffer, A.P. O'Connell, I. Fourel, C.E. Aldrich, and A.R. Jilbert, Hepatology 19:393-411, 1994). The present study was performed to determine the kinetics of disappearance of infected hepatocytes and to evaluate the role of hepatocyte turnover in this process. Essentially all hepatocytes were infected before drug therapy. Oral treatment with 2'-CDG resulted in a prompt reduction in the number of infected hepatocytes. After 2 weeks, only 30 to 50% appeared to still be infected, and less than 10% were detectably infected after 5 weeks of therapy. To assess the possible role of hepatocyte turnover in these changes, 5-bromo-2'-deoxyuridine (BUdR) was administered 8 h before liver biopsy to label host DNA in hepatocytes passing through S phase, and stained nuclei were detected in tissue sections by using an antibody reactive to BUdR. The extent of nuclear labeling after 5 weeks was the same as that before therapy (ca. 1%). However, biopsies taken after 2 weeks of therapy showed a ca. 10-fold elevation in the number of nuclei labeled with BUdR. This result suggested that a rapid clearance of infected hepatocytes by 2'-CDG was caused not just by the inhibition of viral replication but also by an acceleration of the rate of hepatocyte turnover. To test this possibility further, antiviral therapy was carried out with another strong inhibitor of DHBV DNA synthesis, 5-fluoro-2',3'-dideoxy-3'-thiacytidine (524W), which did not accelerate hepatocyte turnover in ducks. 524W administration led to a strong inhibition of virus production but to a slower rate of decline in the number of infected hepatocytes, so that ca. 50% (and perhaps more) were still infected after 3 months of therapy. In addition, histopathologic evaluation of 2'-CDG-treated ducks revealed liver injury, especially at the start of therapy. No liver damage was observed during 524W therapy. These results imply that clearance of infected hepatocytes from the liver is correlated with hepatocyte turnover. Thus, in the absence of immune clearance or other sources for the accelerated elimination of infected hepatocytes, inhibitors of virus replication would have to be administered for a long period to substantially reduce the burden of infected hepatocytes in the liver.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carp N. Z., Saputelli J., Halbherr T. C., Mason W. S., Jilbert A. R. A technique for liver biopsy performed in Pekin ducks using anesthesia with Telazol. Lab Anim Sci. 1991 Oct;41(5):474–475. [PubMed] [Google Scholar]
- Civitico G. M., Locarnini S. A. The half-life of duck hepatitis B virus supercoiled DNA in congenitally infected primary hepatocyte cultures. Virology. 1994 Aug 15;203(1):81–89. doi: 10.1006/viro.1994.1457. [DOI] [PubMed] [Google Scholar]
- Condreay L. D., Jansen R. W., Powdrill T. F., Johnson L. C., Selleseth D. W., Paff M. T., Daluge S. M., Painter G. R., Furman P. A., Ellis M. N. Evaluation of the potent anti-hepatitis B virus agent (-) cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in a novel in vivo model. Antimicrob Agents Chemother. 1994 Mar;38(3):616–619. doi: 10.1128/aac.38.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Davis M., Liotta D. C., Paff M., Frick L. W., Nelson D. J., Dornsife R. E., Wurster J. A., Wilson L. J., Fyfe J. A. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (-) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992 Dec;36(12):2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L. G., Ando K., Hobbs M. V., Ishikawa T., Runkel L., Schreiber R. D., Chisari F. V. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L. G., Guilhot S., Chisari F. V. Interleukin-2 and alpha/beta interferon down-regulate hepatitis B virus gene expression in vivo by tumor necrosis factor-dependent and -independent pathways. J Virol. 1994 Mar;68(3):1265–1270. doi: 10.1128/jvi.68.3.1265-1270.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L. G., Martinez V., Loh Y. T., Rogler C. E., Chisari F. V. Hepatitis B virus nucleocapsid particles do not cross the hepatocyte nuclear membrane in transgenic mice. J Virol. 1994 Sep;68(9):5469–5475. doi: 10.1128/jvi.68.9.5469-5475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhot S., Guidotti L. G., Chisari F. V. Interleukin-2 downregulates hepatitis B virus gene expression in transgenic mice by a posttranscriptional mechanism. J Virol. 1993 Dec;67(12):7444–7449. doi: 10.1128/jvi.67.12.7444-7449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Summers J. pet, a small sequence distal to the pregenome cap site, is required for expression of the duck hepatitis B virus pregenome. J Virol. 1994 Mar;68(3):1564–1572. doi: 10.1128/jvi.68.3.1564-1572.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Johnson L. C., Averett D. R. High-capacity in vitro assessment of anti-hepatitis B virus compound selectivity by a virion-specific polymerase chain reaction assay. Antimicrob Agents Chemother. 1993 Mar;37(3):441–447. doi: 10.1128/aac.37.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilbert A. R., Wu T. T., England J. M., Hall P. M., Carp N. Z., O'Connell A. P., Mason W. S. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992 Mar;66(3):1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajino K., Jilbert A. R., Saputelli J., Aldrich C. E., Cullen J., Mason W. S. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994 Sep;68(9):5792–5803. doi: 10.1128/jvi.68.9.5792-5803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassianides C., Hoofnagle J. H., Miller R. H., Doo E., Ford H., Broder S., Mitsuya H. Inhibition of duck hepatitis B virus replication by 2',3'-dideoxycytidine. A potent inhibitor of reverse transcriptase. Gastroenterology. 1989 Nov;97(5):1275–1280. doi: 10.1016/0016-5085(89)91699-5. [DOI] [PubMed] [Google Scholar]
- Korba B. E., Gerin J. L. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antiviral Res. 1992 Jul 1;19(1):55–70. doi: 10.1016/0166-3542(92)90056-b. [DOI] [PubMed] [Google Scholar]
- Lee B., Luo W. X., Suzuki S., Robins M. J., Tyrrell D. L. In vitro and in vivo comparison of the abilities of purine and pyrimidine 2',3'-dideoxynucleosides to inhibit duck hepadnavirus. Antimicrob Agents Chemother. 1989 Mar;33(3):336–339. doi: 10.1128/aac.33.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscombe C., Pedersen J., Bowden S., Locarnini S. Alterations in intrahepatic expression of duck hepatitis B viral markers with ganciclovir chemotherapy. Liver. 1994 Aug;14(4):182–192. doi: 10.1111/j.1600-0676.1994.tb00072.x. [DOI] [PubMed] [Google Scholar]
- MACDONALD R. A. "Lifespan" of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch Intern Med. 1961 Mar;107:335–343. doi: 10.1001/archinte.1961.03620030023003. [DOI] [PubMed] [Google Scholar]
- Marion P. L., Oshiro L. S., Regnery D. C., Scullard G. H., Robinson W. S. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Cullen J., Saputelli J., Wu T. T., Liu C., London W. T., Lustbader E., Schaffer P., O'Connell A. P., Fourel I. Characterization of the antiviral effects of 2' carbodeoxyguanosine in ducks chronically infected with duck hepatitis B virus. Hepatology. 1994 Feb;19(2):398–411. [PubMed] [Google Scholar]
- Paff M. T., Averett D. R., Prus K. L., Miller W. H., Nelson D. J. Intracellular metabolism of (-)- and (+)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in HepG2 derivative 2.2.15 (subclone P5A) cells. Antimicrob Agents Chemother. 1994 Jun;38(6):1230–1238. doi: 10.1128/aac.38.6.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrillo R. P., Schiff E. R., Davis G. L., Bodenheimer H. C., Jr, Lindsay K., Payne J., Dienstag J. L., O'Brien C., Tamburro C., Jacobson I. M. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med. 1990 Aug 2;323(5):295–301. doi: 10.1056/NEJM199008023230503. [DOI] [PubMed] [Google Scholar]
- Price P. M., Banerjee R., Acs G. Inhibition of the replication of hepatitis B virus by the carbocyclic analogue of 2'-deoxyguanosine. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8541–8544. doi: 10.1073/pnas.86.21.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. M., Banerjee R., Jeffrey A. M., Acs G. The mechanism of inhibition of hepatitis B virus replication by the carbocyclic analog of 2'-deoxyguanosine. Hepatology. 1992 Jul;16(1):8–12. doi: 10.1002/hep.1840160103. [DOI] [PubMed] [Google Scholar]
- Shaw T., Amor P., Civitico G., Boyd M., Locarnini S. In vitro antiviral activity of penciclovir, a novel purine nucleoside, against duck hepatitis B virus. Antimicrob Agents Chemother. 1994 Apr;38(4):719–723. doi: 10.1128/aac.38.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E., Martin J. L., Borroto-Esoda K., Hopkins S., Painter G., Liotta D. C., Furman P. A. The 5'-triphosphates of the (-) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolane-5-yl]cytosine equally inhibit human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993 Aug;37(8):1720–1722. doi: 10.1128/aac.37.8.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. T., Wong S. W., Fung Y. K., Ou J. H. Cell cycle regulation of nuclear localization of hepatitis B virus core protein. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6459–6463. doi: 10.1073/pnas.90.14.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]