Abstract
The virion basic phosphoprotein (BPP), UL32, of the human cytomegalovirus (HCMV) is a 149-kDa tegument protein that represents about 15% of the virion protein mass and is modified by O-linked N-acetylglucosamine (O-GlcNAc). O-GlcNAc has been postulated to mediate subunit-subunit interaction in many different types of intracellular protein complexes, while BPP may play a role in viral assembly and/or envelopment. This report describes the identification of the major O-GlcNAc attachment sites on the HCMV (AD169) BPP. Because the amount of BPP isolated from infectious virions was insufficient to determine the site(s) of glycosylation, the full-length protein has been characterized following overexpression in recombinant baculovirus-infected insect cells. The recombinant protein (rBPP) was electrophoretically (by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and immunologically (by Western immunoassaying) indistinguishable from the BPP isolated from HCMV virions. In addition, the rBPP was modified by O-GlcNAc, and a comparison of the tryptic glycopeptides from the rBPP and native virion BPP indicated that their O-GlcNAc sites are the same. Furthermore, the major sites of O-GlcNAc attachment to the rBPP were mapped on high-performance liquid chromatography-purified glycopeptides by gas phase microsequencing, manual Edman degradation, and electrospray-mass spectrometry. The results demonstrate that the major sites of O-GlcNAc attachment to the BPP are Ser-921 and Ser-952. Identification of these sites will now enable mutagenesis studies to determine the influence of O-GlcNAc on the intracellular location, protein-protein interaction, and biological function of BPP. Finally, the fidelity of the addition of O-GlcNAc to rBPP in insect cells compared with native virion BPP is documented to demonstrate the possible general applicability of the baculovirus expression system to study O-GlcNAc on other low-abundance proteins.
Full text
PDF



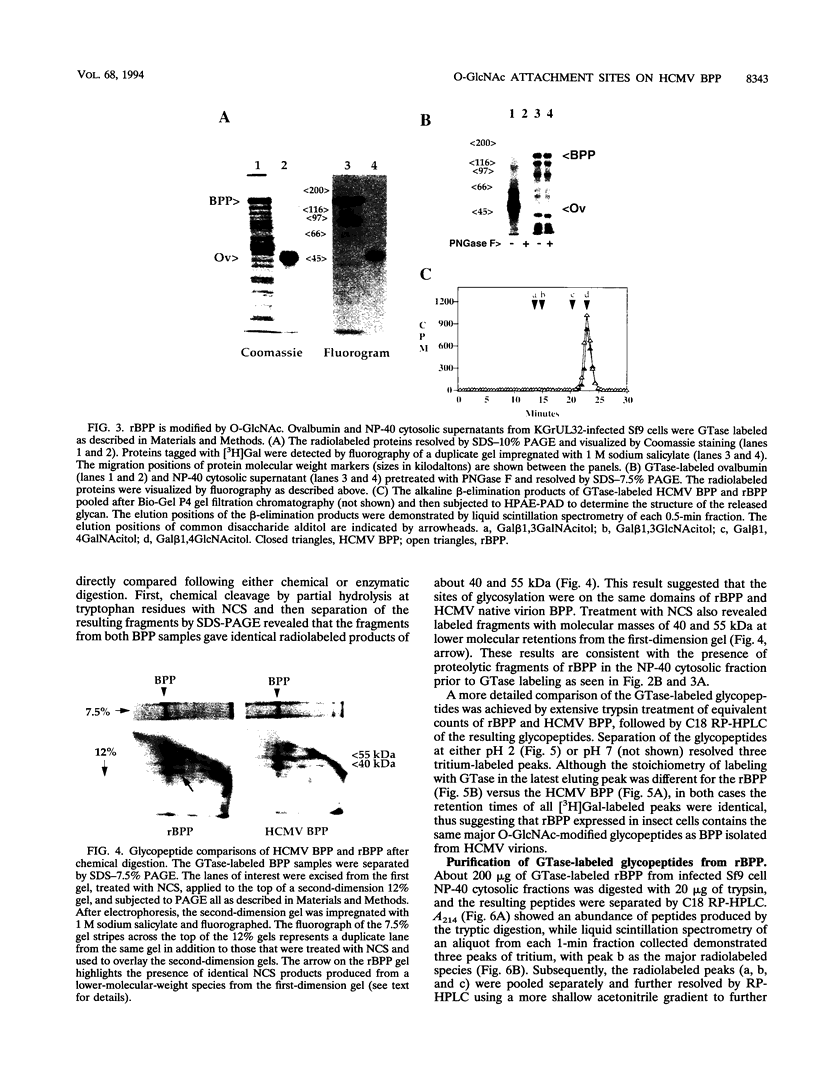



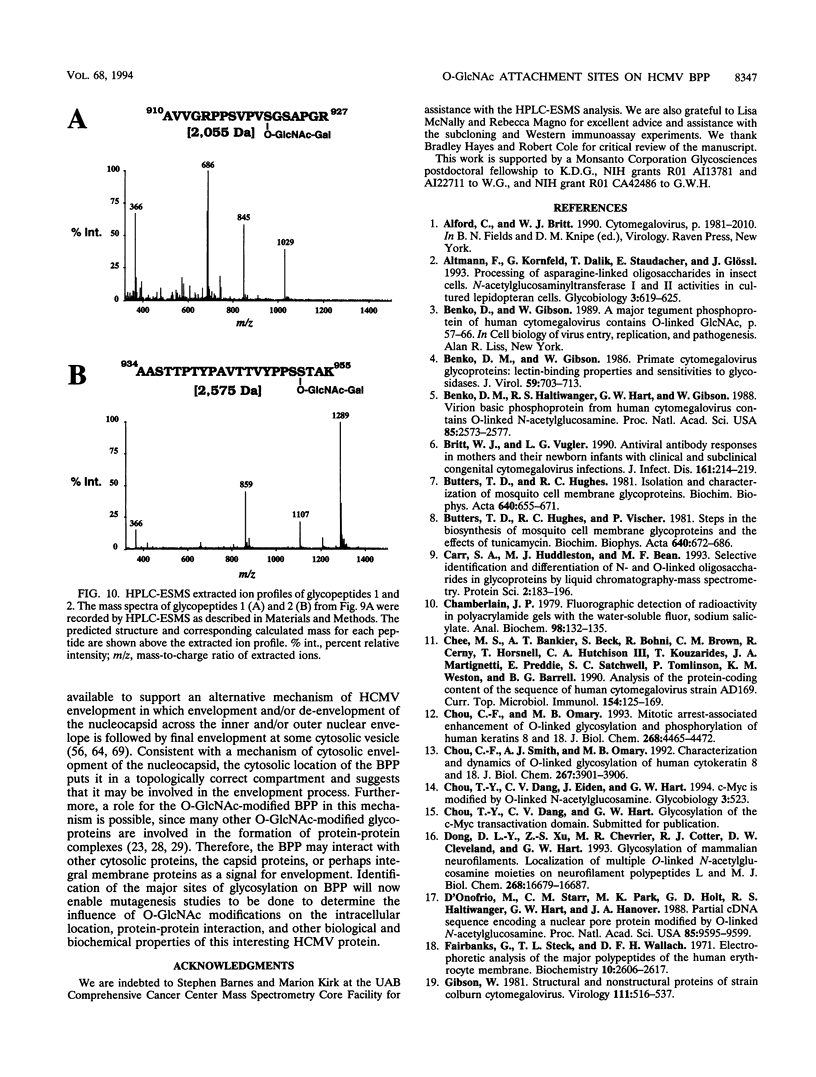


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann F., Kornfeld G., Dalik T., Staudacher E., Glössl J. Processing of asparagine-linked oligosaccharides in insect cells. N-acetylglucosaminyltransferase I and II activities in cultured lepidopteran cells. Glycobiology. 1993 Dec;3(6):619–625. doi: 10.1093/glycob/3.6.619. [DOI] [PubMed] [Google Scholar]
- Benko D. M., Gibson W. Primate cytomegalovirus glycoproteins: lectin-binding properties and sensitivities to glycosidases. J Virol. 1986 Sep;59(3):703–713. doi: 10.1128/jvi.59.3.703-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko D. M., Haltiwanger R. S., Hart G. W., Gibson W. Virion basic phosphoprotein from human cytomegalovirus contains O-linked N-acetylglucosamine. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2573–2577. doi: 10.1073/pnas.85.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Vugler L. G. Antiviral antibody responses in mothers and their newborn infants with clinical and subclinical congenital cytomegalovirus infections. J Infect Dis. 1990 Feb;161(2):214–219. doi: 10.1093/infdis/161.2.214. [DOI] [PubMed] [Google Scholar]
- Butters T. D., Hughes R. C. Isolation and characterization of mosquito cell membrane glycoproteins. Biochim Biophys Acta. 1981 Feb 6;640(3):655–671. doi: 10.1016/0005-2736(81)90096-1. [DOI] [PubMed] [Google Scholar]
- Butters T. D., Hughes R. C., Vischer P. Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim Biophys Acta. 1981 Feb 6;640(3):672–686. doi: 10.1016/0005-2736(81)90097-3. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Huddleston M. J., Bean M. F. Selective identification and differentiation of N- and O-linked oligosaccharides in glycoproteins by liquid chromatography-mass spectrometry. Protein Sci. 1993 Feb;2(2):183–196. doi: 10.1002/pro.5560020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chou C. F., Omary M. B. Mitotic arrest-associated enhancement of O-linked glycosylation and phosphorylation of human keratins 8 and 18. J Biol Chem. 1993 Feb 25;268(6):4465–4472. [PubMed] [Google Scholar]
- Chou C. F., Smith A. J., Omary M. B. Characterization and dynamics of O-linked glycosylation of human cytokeratin 8 and 18. J Biol Chem. 1992 Feb 25;267(6):3901–3906. [PubMed] [Google Scholar]
- D'Onofrio M., Starr C. M., Park M. K., Holt G. D., Haltiwanger R. S., Hart G. W., Hanover J. A. Partial cDNA sequence encoding a nuclear pore protein modified by O-linked N-acetylglucosamine. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9595–9599. doi: 10.1073/pnas.85.24.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D. L., Xu Z. S., Chevrier M. R., Cotter R. J., Cleveland D. W., Hart G. W. Glycosylation of mammalian neurofilaments. Localization of multiple O-linked N-acetylglucosamine moieties on neurofilament polypeptides L and M. J Biol Chem. 1993 Aug 5;268(22):16679–16687. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981 Jun;111(2):516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Gonczol E., Ianacone J., Ho W. Z., Starr S., Meignier B., Plotkin S. Isolated gA/gB glycoprotein complex of human cytomegalovirus envelope induces humoral and cellular immune-responses in human volunteers. Vaccine. 1990 Apr;8(2):130–136. doi: 10.1016/0264-410x(90)90135-9. [DOI] [PubMed] [Google Scholar]
- Haltiwanger R. S., Blomberg M. A., Hart G. W. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1992 May 5;267(13):9005–9013. [PubMed] [Google Scholar]
- Haltiwanger R. S., Hart G. W. Glycosyltransferases as tools in cell biological studies. Methods Mol Biol. 1993;14:175–187. doi: 10.1385/0-89603-226-4:175. [DOI] [PubMed] [Google Scholar]
- Haltiwanger R. S., Kelly W. G., Roquemore E. P., Blomberg M. A., Dong L. Y., Kreppel L., Chou T. Y., Hart G. W. Glycosylation of nuclear and cytoplasmic proteins is ubiquitous and dynamic. Biochem Soc Trans. 1992 May;20(2):264–269. doi: 10.1042/bst0200264. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Cohen C. K., Willingham M. C., Park M. K. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987 Jul 15;262(20):9887–9894. [PubMed] [Google Scholar]
- Holt G. D., Snow C. M., Senior A., Haltiwanger R. S., Gerace L., Hart G. W. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987 May;104(5):1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt G. D., Swiedler S. J., Freed J. H., Hart G. W. Murine Ia-associated invariant chain's processing to complex oligosaccharide forms and its dissociation from the I-Ak complex. J Immunol. 1985 Jul;135(1):399–407. [PubMed] [Google Scholar]
- Hsieh P., Robbins P. W. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984 Feb 25;259(4):2375–2382. [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983 Oct 15;130(1):118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jahn G., Kouzarides T., Mach M., Scholl B. C., Plachter B., Traupe B., Preddie E., Satchwell S. C., Fleckenstein B., Barrell B. G. Map position and nucleotide sequence of the gene for the large structural phosphoprotein of human cytomegalovirus. J Virol. 1987 May;61(5):1358–1367. doi: 10.1128/jvi.61.5.1358-1367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse K. P., Hart G. W. Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1701–1705. doi: 10.1073/pnas.88.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Dahmus M. E., Hart G. W. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem. 1993 May 15;268(14):10416–10424. [PubMed] [Google Scholar]
- Ku N. O., Omary M. B. Expression, glycosylation, and phosphorylation of human keratins 8 and 18 in insect cells. Exp Cell Res. 1994 Mar;211(1):24–35. doi: 10.1006/excr.1994.1054. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Mirolo G., Coppolecchia P., Re M. C., La Placa M. Serum antibodies to individual cytomegalovirus structural polypeptides in renal transplant recipients during viral infection. Microbiol Immunol. 1986;30(7):683–695. doi: 10.1111/j.1348-0421.1986.tb02994.x. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Re M. C., Mirolo G., Baldassarri B., La Placa M. Human immune response to cytomegalovirus structural polypeptides studied by immunoblotting. J Med Virol. 1985 Dec;17(4):303–311. doi: 10.1002/jmv.1890170403. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Ripalti A., Sra K., Pouletty P. Human cytomegalovirus structural proteins: immune reaction against pp150 synthetic peptides. J Clin Microbiol. 1991 Sep;29(9):1868–1872. doi: 10.1128/jcm.29.9.1868-1872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Mach M., Stamminger T., Jahn G. Human cytomegalovirus: recent aspects from molecular biology. J Gen Virol. 1989 Dec;70(Pt 12):3117–3146. doi: 10.1099/0022-1317-70-12-3117. [DOI] [PubMed] [Google Scholar]
- Meek J. L. Prediction of peptide retention times in high-pressure liquid chromatography on the basis of amino acid composition. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1632–1636. doi: 10.1073/pnas.77.3.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Mullis K. G., Haltiwanger R. S., Hart G. W., Marchase R. B., Engler J. A. Relative accessibility of N-acetylglucosamine in trimers of the adenovirus types 2 and 5 fiber proteins. J Virol. 1990 Nov;64(11):5317–5323. doi: 10.1128/jvi.64.11.5317-5323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro D., Paz P., Tugizov S., Topp K., La Vail J., Pereira L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993 Nov;197(1):143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- Novelli A., Boulanger P. A. Deletion analysis of functional domains in baculovirus-expressed adenovirus type 2 fiber. Virology. 1991 Nov;185(1):365–376. doi: 10.1016/0042-6822(91)90784-9. [DOI] [PubMed] [Google Scholar]
- Park M. K., D'Onofrio M., Willingham M. C., Hanover J. A. A monoclonal antibody against a family of nuclear pore proteins (nucleoporins): O-linked N-acetylglucosamine is part of the immunodeterminant. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6462–6466. doi: 10.1073/pnas.84.18.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachter B., Wieczorek L., Scholl B. C., Ziegelmaier R., Jahn G. Detection of cytomegalovirus antibodies by an enzyme-linked immunosorbent assay using recombinant polypeptides of the large phosphorylated tegument protein pp150. J Clin Microbiol. 1992 Jan;30(1):201–206. doi: 10.1128/jcm.30.1.201-206.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky M. L. A subpopulation of the avian erythroblastosis virus v-erbA protein, a member of the nuclear hormone receptor family, is glycosylated. J Virol. 1990 Jan;64(1):463–466. doi: 10.1128/jvi.64.1.463-466.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radsak K. D., Brücher K. H., Georgatos S. D. Focal nuclear envelope lesions and specific nuclear lamin A/C dephosphorylation during infection with human cytomegalovirus. Eur J Cell Biol. 1991 Apr;54(2):299–304. [PubMed] [Google Scholar]
- Rasmussen L., Mullenax J., Nelson M., Merigan T. C. Human cytomegalovirus polypeptides stimulate neutralizing antibody in vivo. Virology. 1985 Aug;145(1):186–190. doi: 10.1016/0042-6822(85)90215-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Nelson M., Neff M., Merigan T. C., Jr Characterization of two different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology. 1988 Apr;163(2):308–318. doi: 10.1016/0042-6822(88)90271-1. [DOI] [PubMed] [Google Scholar]
- Reason A. J., Morris H. R., Panico M., Marais R., Treisman R. H., Haltiwanger R. S., Hart G. W., Kelly W. G., Dell A. Localization of O-GlcNAc modification on the serum response transcription factor. J Biol Chem. 1992 Aug 25;267(24):16911–16921. [PubMed] [Google Scholar]
- Roby C., Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986 Sep;59(3):714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquemore E. P., Chou T. Y., Hart G. W. Detection of O-linked N-acetylglucosamine (O-GlcNAc) on cytoplasmic and nuclear proteins. Methods Enzymol. 1994;230:443–460. doi: 10.1016/0076-6879(94)30028-3. [DOI] [PubMed] [Google Scholar]
- Scholl B. C., Von Hintzenstern J., Borisch B., Traupe B., Bröker M., Jahn G. Prokaryotic expression of immunogenic polypeptides of the large phosphoprotein (pp150) of human cytomegalovirus. J Gen Virol. 1988 Jun;69(Pt 6):1195–1204. doi: 10.1099/0022-1317-69-6-1195. [DOI] [PubMed] [Google Scholar]
- Severi B., Landini M. P., Govoni E. Human cytomegalovirus morphogenesis: an ultrastructural study of the late cytoplasmic phases. Arch Virol. 1988;98(1-2):51–64. doi: 10.1007/BF01321005. [DOI] [PubMed] [Google Scholar]
- Snow C. M., Senior A., Gerace L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol. 1987 May;104(5):1143–1156. doi: 10.1083/jcb.104.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr C. M., Hanover J. A. Glycosylation of nuclear pore protein p62. Reticulocyte lysate catalyzes O-linked N-acetylglucosamine addition in vitro. J Biol Chem. 1990 Apr 25;265(12):6868–6873. [PubMed] [Google Scholar]
- Sullivan S., Wong T. W. A manual sequencing method for identification of phosphorylated amino acids in phosphopeptides. Anal Biochem. 1991 Aug 15;197(1):65–68. doi: 10.1016/0003-2697(91)90356-x. [DOI] [PubMed] [Google Scholar]
- Tooze J., Hollinshead M., Reis B., Radsak K., Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993 Feb;60(1):163–178. [PubMed] [Google Scholar]
- Torres C. R., Hart G. W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984 Mar 10;259(5):3308–3317. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B., Kropff B., Kalbacher H., Britt W., Sundqvist V. A., Ostberg L., Mach M. A continuous sequence of more than 70 amino acids is essential for antibody binding to the dominant antigenic site of glycoprotein gp58 of human cytomegalovirus. J Virol. 1992 Sep;66(9):5290–5297. doi: 10.1128/jvi.66.9.5290-5297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteheart S. W., Passaniti A., Reichner J. S., Holt G. D., Haltiwanger R. S., Hart G. W. Glycosyltransferase probes. Methods Enzymol. 1989;179:82–95. doi: 10.1016/0076-6879(89)79116-3. [DOI] [PubMed] [Google Scholar]
- Whitford M., Faulkner P. A structural polypeptide of the baculovirus Autographa californica nuclear polyhedrosis virus contains O-linked N-acetylglucosamine. J Virol. 1992 Jun;66(6):3324–3329. doi: 10.1128/jvi.66.6.3324-3329.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]






