Abstract
We analyzed whether the phosphotransferase encoded by the UL97 open reading frame of human cytomegalovirus (HCMV) alone is sufficient to confer ganciclovir (GCV) susceptibility to a foreign virus. Two vaccinia virus recombinants (T1 and A5) containing the UL97 open reading frames from a GCV-sensitive HCMV and from a GCV-resistant strain were constructed. T1 exhibited a GCV-sensitive phenotype in plaque reduction assays, whereas A5 did not. Moreover, T1-infected cell cultures showed a strongly increased incorporation of [14C]GCV triphosphate into macromolecular DNA, compared with recombinant A5 or vaccinia virus controls, which could be inhibited by the addition of guanosine. This shows that UL97 kinase is the only additional gene product required to make vaccinia virus susceptible to GCV, and guanosine seems to be one natural substrate for the enzyme. The system described here should be very helpful for fast and detailed functional analyses of UL97 mutations found in GCV-resistant HCMV isolates.
Full text
PDF
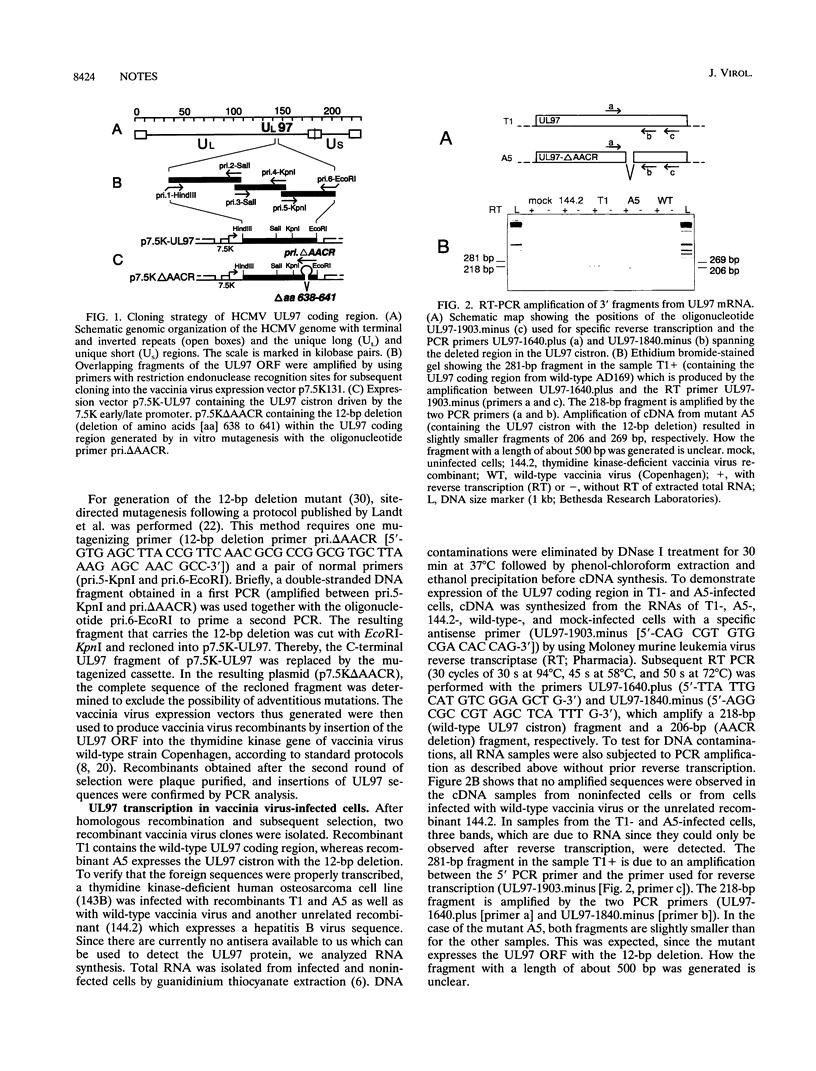
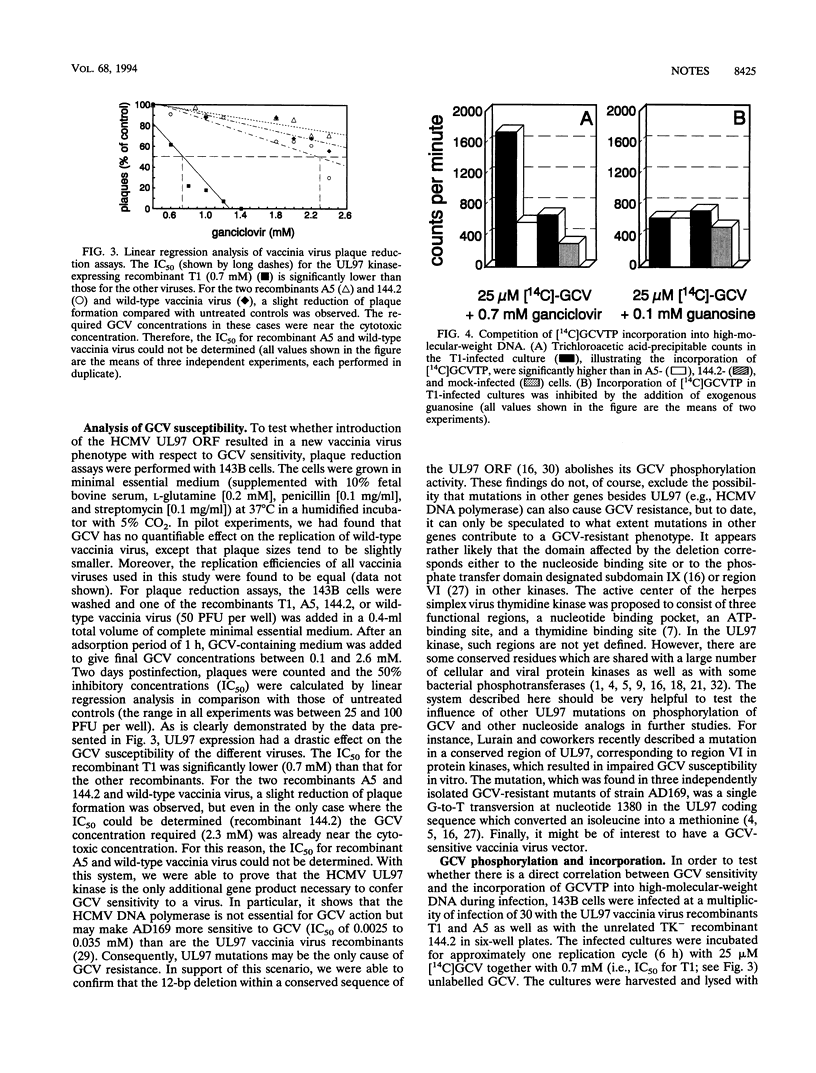


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylis S. A., Banham A. H., Vydelingum S., Dixon L. K., Smith G. L. African swine fever virus encodes a serine protein kinase which is packaged into virions. J Virol. 1993 Aug;67(8):4549–4556. doi: 10.1128/jvi.67.8.4549-4556.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K., Fyfe J. A., Stanat S. C., Leslie L. K., Sorrell J. B., Lambe C. U., Coen D. M. A human cytomegalovirus mutant resistant to the nucleoside analog 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine (BW B759U) induces reduced levels of BW B759U triphosphate. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8769–8773. doi: 10.1073/pnas.83.22.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K., Stanat S. C., Sorrell J. B., Fyfe J. A., Keller P. M., Lambe C. U., Nelson D. J. Metabolic activation of the nucleoside analog 9-[( 2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. Phosphotransferase sequence homology. Nature. 1987 Sep 3;329(6134):21–21. doi: 10.1038/329021a0. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Lawrence G. L., Barrell B. G. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 1989 May;70(Pt 5):1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Drew W. L. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988 Aug;158(2):449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- Drew W. L. Nonpulmonary manifestations of cytomegalovirus infection in immunocompromised patients. Clin Microbiol Rev. 1992 Apr;5(2):204–210. doi: 10.1128/cmr.5.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erice A., Chou S., Biron K. K., Stanat S. C., Balfour H. H., Jr, Jordan M. C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989 Feb 2;320(5):289–293. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- Farrugia E., Schwab T. R. Management and prevention of cytomegalovirus infection after renal transplantation. Mayo Clin Proc. 1992 Sep;67(9):879–890. doi: 10.1016/s0025-6196(12)60828-3. [DOI] [PubMed] [Google Scholar]
- Goodrich J. M., Bowden R. A., Fisher L., Keller C., Schoch G., Meyers J. D. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993 Feb 1;118(3):173–178. doi: 10.7326/0003-4819-118-3-199302010-00003. [DOI] [PubMed] [Google Scholar]
- Graham D., Larder B. A., Inglis M. M. Evidence that the 'active centre' of the herpes simplex virus thymidine kinase involves an interaction between three distinct regions of the polypeptide. J Gen Virol. 1986 Apr;67(Pt 4):753–758. doi: 10.1099/0022-1317-67-4-753. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G., Heffron F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991 Aug 30;253(5023):1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS). Clinical findings, diagnosis, and treatment. Ann Intern Med. 1988 Apr;108(4):585–594. doi: 10.7326/0003-4819-108-4-585. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Drillien R., Spehner D., Skory S., Schmitt D., Wiktor T., Koprowski H., Lecocq J. P. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984 Nov 8;312(5990):163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- Lacasa M. A protein kinase-related gene within the channel catfish herpesvirus genome. Nucleic Acids Res. 1990 May 25;18(10):3050–3050. doi: 10.1093/nar/18.10.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt O., Grunert H. P., Hahn U. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene. 1990 Nov 30;96(1):125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- Littler E., Stuart A. D., Chee M. S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992 Jul 9;358(6382):160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- Lurain N. S., Spafford L. E., Thompson K. D. Mutation in the UL97 open reading frame of human cytomegalovirus strains resistant to ganciclovir. J Virol. 1994 Jul;68(7):4427–4431. doi: 10.1128/jvi.68.7.4427-4431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurain N. S., Thompson K. D., Holmes E. W., Read G. S. Point mutations in the DNA polymerase gene of human cytomegalovirus that result in resistance to antiviral agents. J Virol. 1992 Dec;66(12):7146–7152. doi: 10.1128/jvi.66.12.7146-7152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. M., Horak D. A., Niland J. C., Duncan S. R., Forman S. J., Zaia J. A. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants; The City of Hope-Stanford-Syntex CMV Study Group. N Engl J Med. 1991 Apr 11;324(15):1005–1011. doi: 10.1056/NEJM199104113241501. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J Virol. 1989 Jan;63(1):450–455. doi: 10.1128/jvi.63.1.450-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanat S. C., Reardon J. E., Erice A., Jordan M. C., Drew W. L., Biron K. K. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother. 1991 Nov;35(11):2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Biron K. K., Talarico C., Stanat S. C., Davis M., Pozzi L. M., Coen D. M. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob Agents Chemother. 1993 Jan;37(1):19–25. doi: 10.1128/aac.37.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Talarico C. L., Stanat S. C., Davis M., Coen D. M., Biron K. K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992 Jul 9;358(6382):162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- Tatarowicz W. A., Lurain N. S., Thompson K. D. A ganciclovir-resistant clinical isolate of human cytomegalovirus exhibiting cross-resistance to other DNA polymerase inhibitors. J Infect Dis. 1992 Oct;166(4):904–907. doi: 10.1093/infdis/166.4.904. [DOI] [PubMed] [Google Scholar]
- Traktman P., Anderson M. K., Rempel R. E. Vaccinia virus encodes an essential gene with strong homology to protein kinases. J Biol Chem. 1989 Dec 25;264(36):21458–21461. [PubMed] [Google Scholar]
- Winston D. J., Ho W. G., Bartoni K., Du Mond C., Ebeling D. F., Buhles W. C., Champlin R. E. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med. 1993 Feb 1;118(3):179–184. doi: 10.7326/0003-4819-118-3-199302010-00004. [DOI] [PubMed] [Google Scholar]
- de Wind N., Domen J., Berns A. Herpesviruses encode an unusual protein-serine/threonine kinase which is nonessential for growth in cultured cells. J Virol. 1992 Sep;66(9):5200–5209. doi: 10.1128/jvi.66.9.5200-5209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Salle H., Altenburger W., Elkaim R., Dott K., Dieterlé A., Drillien R., Cazenave J. P., Tolstoshev P., Lecocq J. P. Active gamma-carboxylated human factor IX expressed using recombinant DNA techniques. Nature. 1985 Jul 18;316(6025):268–270. doi: 10.1038/316268a0. [DOI] [PubMed] [Google Scholar]




