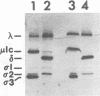
Abstract
Reovirus adheres specifically to apical membranes of mouse intestinal M cells and exploits M-cell transepithelial transport activity to enter Peyer's patch mucosa, where replication occurs. Proteolytic conversion of native reovirus to intermediate subviral particles (ISVPs) occurs in the intestine, but it is not known whether conversion is essential for interaction of virus with M cells. We tested the capacity of native virions, ISVPs, and cores (that lack outer capsid proteins) to bind to intestinal epithelial cells in vivo and found that only ISVPs adhered to M cells. Thus, intraluminal conversion of native reovirus to ISVPs is a prerequisite for M-cell adherence, and outer capsid proteins unique to ISVPs (either sigma 1 or products of mu 1) mediate interaction of virus with M-cell apical membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amerongen H. M., Weltzin R., Farnet C. M., Michetti P., Haseltine W. A., Neutra M. R. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4(8):760–765. [PubMed] [Google Scholar]
- Bass D. M., Bodkin D., Dambrauskas R., Trier J. S., Fields B. N., Wolf J. L. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J Virol. 1990 Apr;64(4):1830–1833. doi: 10.1128/jvi.64.4.1830-1833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin D. K., Nibert M. L., Fields B. N. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989 Nov;63(11):4676–4681. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- Dryden K. A., Wang G., Yeager M., Nibert M. L., Coombs K. M., Furlong D. B., Fields B. N., Baker T. S. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J Cell Biol. 1993 Sep;122(5):1023–1041. doi: 10.1083/jcb.122.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong D. B., Nibert M. L., Fields B. N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988 Jan;62(1):246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Neutra M. R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992 Oct;72(4):853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Leone G., Maybaum L., Lee P. W. The reovirus cell attachment protein possesses two independently active trimerization domains: basis of dominant negative effects. Cell. 1992 Oct 30;71(3):479–488. doi: 10.1016/0092-8674(92)90516-f. [DOI] [PubMed] [Google Scholar]
- Lucia-Jandris P., Hooper J. W., Fields B. N. Reovirus M2 gene is associated with chromium release from mouse L cells. J Virol. 1993 Sep;67(9):5339–5345. doi: 10.1128/jvi.67.9.5339-5345.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutra M. R., Kraehenbuhl J. P. Transepithelial transport and mucosal defence I: the role of M cells. Trends Cell Biol. 1992 May;2(5):134–138. doi: 10.1016/0962-8924(92)90099-9. [DOI] [PubMed] [Google Scholar]
- Nibert M. L., Dermody T. S., Fields B. N. Structure of the reovirus cell-attachment protein: a model for the domain organization of sigma 1. J Virol. 1990 Jun;64(6):2976–2989. doi: 10.1128/jvi.64.6.2976-2989.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M. L., Fields B. N. A carboxy-terminal fragment of protein mu 1/mu 1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J Virol. 1992 Nov;66(11):6408–6418. doi: 10.1128/jvi.66.11.6408-6418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M. L., Furlong D. B., Fields B. N. Mechanisms of viral pathogenesis. Distinct forms of reoviruses and their roles during replication in cells and host. J Clin Invest. 1991 Sep;88(3):727–734. doi: 10.1172/JCI115369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M. L., Schiff L. A., Fields B. N. Mammalian reoviruses contain a myristoylated structural protein. J Virol. 1991 Apr;65(4):1960–1967. doi: 10.1128/jvi.65.4.1960-1967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Ermak T. H. Structural specializations for antigen uptake and processing in the digestive tract. Springer Semin Immunopathol. 1990;12(2-3):139–152. doi: 10.1007/BF00197502. [DOI] [PubMed] [Google Scholar]
- Roivainen M., Huovilainen A., Hovi T. Antigenic modification of polioviruses by host proteolytic enzymes. Arch Virol. 1990;111(1-2):115–125. doi: 10.1007/BF01310509. [DOI] [PubMed] [Google Scholar]
- Siciński P., Rowiński J., Warchoł J. B., Jarzabek Z., Gut W., Szczygieł B., Bielecki K., Koch G. Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology. 1990 Jan;98(1):56–58. doi: 10.1016/0016-5085(90)91290-m. [DOI] [PubMed] [Google Scholar]
- Sturzenbecker L. J., Nibert M., Furlong D., Fields B. N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987 Aug;61(8):2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M. T., Nibert M. L., Fields B. N. Ion channels induced in lipid bilayers by subvirion particles of the nonenveloped mammalian reoviruses. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10549–10552. doi: 10.1073/pnas.90.22.10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K. L., Bronson R. T., Byers K. B., Fields B. Molecular basis of viral neurotropism: experimental reovirus infection. Neurology. 1985 Jan;35(1):88–92. doi: 10.1212/wnl.35.1.88. [DOI] [PubMed] [Google Scholar]
- Weltzin R., Lucia-Jandris P., Michetti P., Fields B. N., Kraehenbuhl J. P., Neutra M. R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989 May;108(5):1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. L., Rubin D. H., Finberg R., Kauffman R. S., Sharpe A. H., Trier J. S., Fields B. N. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981 Apr 24;212(4493):471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]