Abstract
A 3-yr-old female patient exhibited interleukin 12 (IL-12) deficiency that was associated with recurrent episodes of pneumococcal pneumonia with sepsis and other infections in the absence of fevers. The patient’s peripheral blood mononuclear cells (PBMCs) exhibited normal proliferative responses to antigens. Immune responses, including in vivo production of antibodies to diphtheria, tetanus, or pneumococcal antigens, were normal. Ig levels and B cell and T cell phenotypes were also normal. In contrast, IL-12 p70 heterodimer production was undetectable by using supernatants of the patient’s stimulated PBMCs when compared with control cells treated similarly. Although present, interferon γ (IFN-γ) was reduced. The addition of recombinant IFN-γ to control cells enhanced the production of IL-12 by up to sixfold. By contrast, IL-12 was undetectable in supernatants of the patient’s cells in the presence of recombinant IFN-γ. IL-12 p40 subunit mRNA by using the patient’s PBMCs after stimulation with Staphylococcus aureus Cowan strain 1 or lipopolysaccharide was also undetectable by reverse transcription–PCR when compared with control cells. Production of IL-2, IL-6, tumor necrosis factor α, or IFN-γ of the patient’s PBMCs after appropriate stimulation was observed. This patient has either a defect in Staphylococcus aureus Cowan strain 1-lipopolysaccharide- or staphylococcal enterotoxin A-induced signaling pathways for the activation of IL-12 p40 gene expression, or an abnormality in the IL-12 p40 gene itself.
Keywords: immunodeficiency/pneumonia/interleukin 12
Primary immunodeficiency diseases are attributable to a deficiency in cellular, humoral, or molecular functions of the rather complex immunity systems. Patients with primary immunodeficiency diseases usually present clinically with an increased frequency, severity, or persistence of infections or with an occurrence of infections that are attributable to opportunistic pathogens. Although >50 well-defined generally genetically determined primary immunodeficiencies and a similar number of complex syndromes in which immunodeficiency represents but one component have been already defined, the precise basis for the immunodeficiency of many patients who exhibit increased susceptibility to infections or present with unusual infections has remained elusive. Cells of normal immune systems respond to products of bacterial or other intracellular or extracellular pathogens by producing a variety of cytokines that are crucial to bodily defenses. Thus, evaluation of the cytokine profiles by using the cells or serum of patients with apparent immunodeficiency represents one logical approach to elucidate the mechanisms of unexplained immunodeficiency states.
Immunodeficiencies that are attributable to cytokine defects to date include a deficiency of expression of interleukin 2 (IL-2) and IL-2 mRNA (1, 2), multiple cytokine deficiencies that include IL-2, IL-4, IL-5, and interferon γ (IFN-γ); these cytokine deficiencies are attributed to abnormalities of the nuclear factor of activated T cells (NF-AT) in the promotor region of IL-2 (3), and a deficiency of the receptor for IFN-γ (4–8) or IL-12 (9, 10).
Herein, we present the results of an investigation of cytokine production and cytokine gene expression by using cells from a child who suffered from recurrent pneumonia and frequent, recurrent infections caused by high-grade pyogenic bacterial pathogens, including Streptococcus pneumoniae or Staphylococcus aureus, but who did not exhibit fevers. A comprehensive evaluation of the specific immunoparameters known to be associated with increased susceptibility to infection with these pathogens failed to reveal a basis for the immunodeficiency of this child. Instead, we found that this patient exhibited a profound deficiency in the expression and production of IL-12.
IL-12, a heterodimeric cytokine, is comprised of two subunits, p40 and p35 (11–14). This cytokine is produced mainly by monocytes/macrophages and promotes the development and activity of cytotoxic T lymphocytes, natural killer cells, lymphokine-activated killer cells, and macrophages; consequently, IL-12 may participate in host defenses against a variety of microbial pathogens (15–17). IL-12 is potentially a vital regulator of the cytokine network and as such may represent an important mediator of the outcome of infectious diseases (15–17). Therefore, it is attractive to consider failure of IL-12 production as a possible basis of primary immunodeficiency in humans, and in this patient in particular.
CASE REPORT
The patient KC, a product of an incestuous relationship, is a 3-yr-old girl born to a 15-yr-old mother. At 5 wk after birth, the child was hospitalized for buccal cellulitis, at which time infection with S. aureus was present at the site of the cellulitis. At 2 mo of age, the patient was hospitalized for cervical lymphadenitis. At 10 mo of age, the patient had severe impetigo without fever. At 11 mo, she was hospitalized again for a pulmonary infection, and S. pneumoniae was cultured from the blood. She was hospitalized 2 mo later for septic arthritis of the left hip and also at 15 mo of age for cellulitis of the right big toe. Both of these admissions were associated with pneumococcal infections, and the etiology was established. At 17 mo and again at 19 mo, the child was hospitalized for pneumonia and then for cervical lymphadenitis, but blood cultures on these occasions were negative. Most recently, the child has had an additional septic infection associated with rather impressive lymphadenitis; S. pneumoniae was again cultured from the blood (Table 1). Laboratory studies, including a rather comprehensive evaluation of immunological parameters, were normal, with the exception that she exhibited an impressive and consistent deficiency in the production of the cytokine IL-12.
Table 1.
History of infections
| Age | Infections |
|---|---|
| 5 wk | Buccal cellulitis, sepsis (S. aureus), and severe thrush |
| 2 mo | Cervical lymphadentis |
| 10 mo | Severe impetigo |
| 11 mo | Pneumonia and sepsis (pneumococcus) |
| 13 mo | Septic arthritis of left hip |
| 15 mo | Right big toe cellulitis with sepsis (pneumococcus) |
| 17 mo | Pneumonia (negative cultures) |
| 19 mo | Cervical lymphadenitis |
| 25 mo | Lymphadenitis (pneumococcus) |
MATERIALS AND METHODS
Flow Cytometric Analysis.
The following mAbs (Coulter) were used for flow cytometric analyses: RD1/phycoerythrin-labeled anti-CD2, anti-CD4, anti-CD14, anti-CD56, and control mouse IgG1; PC5-labeled anti-CD3 and control mouse IgG1; fluorescein isothiocyanate-labeled anti-CD8, anti-CD20, and control mouse IgG1; and energy-coupled dye-labeled anti-CD19 and control mouse IgG2b. The cells were analyzed by three-color fluorescence flow cytometry by using an Epics XL-MCL flow cytometer (Coulter).
Proliferative Assays.
The following lectins or antigens (Ags) were used for lymphocyte proliferation assays: phytohemagglutinin (Difco), Con A (Calbiochem), pokeweed mitogen (Life Technologies, Grand Island, NY), tetanus toxoid (Connaught Laboratories), Candida albicans (Greer Labs, Lendir, NC), and fixed S. aureus Cowan strain 1 (SAC) (Pansorbin, Calbiochem). Peripheral blood mononuclear cells (PBMCs) were stimulated with lectins at 1 × 105/200 μl/well or with Ags at 2.5 × 105/200 μl/well in a 96-well U-bottom plate for 48 h and then pulsed with [3H]thymidine (0.5 μCi/well for lectin stimulation, 1.0 μCi/well for Ag stimulation; 1 Ci = 37 GBq; Amersham) for another 18 h.
Immunological Analyses.
Ig levels and IgG subclass levels of Igs (IgG1, IgG2, IgG3, and IgG4) were performed by using standard techniques. Other analyses included pneumococcal, diphtheria, and tetanus antibody titers; phagocytic functions; and complement levels. Determination of HIV was performed by using an HIV DNA–PCR kit (Amplicor; Roche Diagnostics).
Cell Culture.
PBMCs from the patient and a healthy HIV-negative control were isolated by density gradient centrifugation by using Ficoll–Paque Plus (Pharmacia Biotech) and tested at 4 × 105 cells/200 μl/well in a 96-well flat-bottom plate for ELISA or at 10 × 106 cells/5 ml/well in a 6-well plate for RNA analysis in RPMI 1640 medium with 10% fetal bovine serum (HyClone), 100 units/ml penicillin, and 100 μg/ml streptomycin for 24 h in a humidified 5% CO2/95% air incubator. For ELISA, the PBMCs were stimulated with 1:10,000 (wt/vol) of SAC for 24 h, with 10 ng/ml of staphylococcal enterotoxin A (SEA) (Toxin Technology) for 24 and 48 h, or with phorbol 12-myristate 13-acetate (PMA) plus ionomycin (both from Sigma) for 24 h. For RNA analysis, the PBMCs were stimulated with 1:10,000 (wt/vol) of SAC or with 1 μg/ml of lipopolysaccharide (LPS) from Escherichia coli serotype 055:B5 (Sigma) for 4 h.
ELISA.
Supernatants from the cell cultures were harvested, centrifuged to remove cell debris, aliquoted, and stored at −70°C until analyzed. Immunoassay kits from R & D Systems (Quantikine) were used to measure the concentrations of human IL-2, IFN-γ, and IL-12 p70 in the supernatants. The concentrations of IL-6 and tumor necrosis factor α (TNF-α) was measured by using human ELISA kits from Immunotech (Westbrook, ME) and BioSource Europe (Medgenix; Fleurus, Belgium), respectively. The sensitivity of the assay is ≤3 pg/ml for IFN-γ, IL-6, and TNF-α; ≤5 pg/ml for IL-12 p70; and ≤7 pg/ml for IL-2. To determine whether the deficiency of IL-12 was attributable to the reduced IFN-γ observed in the patient’s cells when stimulated with SAC or SEA, experiments were performed in which recombinant human IFN-γ (specific activity of ≥2 × 107 units/mg; PeproTech, Rocky Hill, NJ) was added in vitro to control cells or to the patient’s cells in varying concentrations; the mixtures were incubated for 24 h. Supernatants from the cell cultures were harvested as described above and analyzed for IL-12 p70 by ELISA by using the kits indicated above.
RNA Analysis.
Total cellular RNA was isolated from the cells by using RNAzol (Biotecx Laboratories, Houston) according to the manufacturer’s protocol with modifications. cDNA was made from RNA samples by using Promega’s Reverse Transcription System in a Perkin–Elmer/Cetus 9600 Thermal Cycler. PCR primers specific for human IL-12 p40 and p35 subunits and β-actin were synthesized from Integrated DNA Technologies (Coralville, IA). Their sequences were as follows: IL-12 p40 sense, 5′-TCAAAGAGTTTGGAGATGCTGGCC-3′; IL-12 p40 antisense, 5′-TGATGATGTCCCTGATGAAGAAGC-3′; IL-12 p35 sense, 5′-CCTCCTGGACCACCTCAGTTTG-3′; IL-12 p35 antisense, 5′-GAACTCCACCTGGTACATCTTCAAGTC-3′; β-actin sense, 5′-GTGATGGTGGGCATGGGTCA-3′; and β-actin antisense, 5′-TTAATGTCACGCACGATTTCCC-3′.
PCR was initiated in this thermal cycler programmed for at 95°C for 15 sec, 56°C for 15 sec, and 72°C for 75 sec. To ensure that amplification was terminated while still in the linear phase of the reaction, PCR was performed for 30 cycles. PCR products were analyzed by electrophoresis through a 2% SeaKem GTG agarose (FMC) gel containing ethidium bromide and visualized with a UV light source. The gel was photographed by using Polaroid 665 film. The negative was analyzed by densitometric scanning on the Multiscan-R (Interactive Technologies International, St. Petersburg, FL). The IL-12 band densities were normalized to the corresponding β-actin densities and expressed as arbitrary optical density units.
RESULTS
Immunological Analyses of the Patient.
The Ig levels; pneumococcal, diphtheria, and tetanus antibody titers; phagocytic functions; and complement levels of the patient have been tested on more than three separate occasions since the patient was 3 mo of age. The results of each of the above immunological studies were entirely within normal limits. The patient is HIV-negative as determined by HIV DNA–PCR (data not shown).
Analysis by flow cytometry by using mAbs to CD2 and CD3, CD19 and CD20, CD14, and CD56 performed on multiple occasions showed that the patient’s PBMCs had normal percentages and absolute numbers of T lymphocytes, B lymphocytes, monocytes, and natural killer cells (Table 2). The percentages and absolute numbers of CD4+ or CD8+ T cells in the patient’s PBMCs were also within normal limits (Table 2). Proliferative responses of the patient’s PBMCs to lectins, phytohemagglutinin, Con A, or pokeweek mitogen as well as to Candida or tetanus Ags were within normal limits. However, when PBMCs were stimulated with SAC, the proliferative response of the patient’s PBMCs was significantly higher than that of normal control PBMCs treated in an identical manner (Table 3).
Table 2.
Flow cytometric analysis of PBMCs of patient (KC)
| CD markers | % Positive cells |
|---|---|
| CD2 | 71 |
| CD3 | 68–69 |
| CD4 | 51–54 |
| CD8 | 13–15 |
| CD14 | 17 |
| CD19 | 27–28 |
| CD20 | 25–27 |
| CD56 | 2–3 |
A total of 5,000 cells per sample were analyzed. Results are representative of multiple separate samples.
Table 3.
Proliferative responses of PBMCs of the patient (KC) to Ags or lectins
| Stimulant | [3H]Thymidine incorporation, dpm
|
||
|---|---|---|---|
| KC | control | ||
| SAC | 1:8,000, wt/vol | 70,074 | 13,036 |
| 1:800 | 111,163 | 17,196 | |
| 1:80 | 71,648 | 16,190 | |
| Candida | 4 μg/ml | 12,107 | 30,904 |
| 20 | 18,447 | 39,935 | |
| 100 | 30,379 | 29,943 | |
| Tetanus | 0.1 Lf | 4,855 | 12,741 |
| 0.3 | 5,845 | 24,118 | |
| 0.5 | 10,694 | 25,855 | |
| Medium | 2,331 | 2,081 | |
| Phytohemagglutinin | 1 μg/ml | 133,573 | 190,312 |
| 2 | 249,044 | 247,795 | |
| 4 | 362,278 | 279,132 | |
| Con A | 25 μg/ml | 130,906 | 80,755 |
| 50 | 191,778 | 160,960 | |
| 100 | 202,751 | 166,265 | |
| Pokeweed mitogen | 1:50 | 41,117 | 57,341 |
| Medium | 682 | 793 | |
PBMCs were incubated for 66 h with varying concentrations of Ags or lectins. The proliferative responses were determined in terms of [3H]thymidine uptake into the cells during the last 18 h of culture. Results are expressed as the mean of triplicate cultures. The deviation from the mean is always <10% of the mean value. Results are representative of two separate studies.
Determination of IFN-γ, IL-2, IL-6, TNF-α, and IL-12 After Stimulation with SAC, SEA, and PMA/Ionomycin by Using the Patient’s Cells.
As shown in Table 4, the patient’s PBMCs produced IFN-γ and IL-2 upon stimulation with SEA, although the concentrations of IFN-γ and IL-2 were somewhat lower than those observed in PBMCs of healthy controls. IL-2 was also produced from the patient’s PBMCs upon stimulation with SAC, but the production of IFN-γ by using the patient’s cells stimulated with SAC was not detectable. The patient’s PBMCs produced IL-6 and TNF-α upon stimulation with SAC or SEA; the SEA-induced TNF-α levels of the patient were comparable with those of controls, whereas the SEA-induced IL-6 and SAC-induced TNF-α and IL-6 levels produced by the patient’s cells were significantly lower than those of controls. IFN-γ, IL-2, IL-6, and TNF-α production by the patient’s cells upon activation of bypass signaling pathways with PMA plus ionomycin was identical with the production observed for controls. By contrast, IL-12 was not detectable at any time in these experiments by using a wide variety of stimulants performed on two separate occasions.
Table 4.
Determination of IFN-γ, IL-2, IL-6, and TNF-α with SAC, SEA, or PMA plus ionomycin using the patient’s cells
| Stimulant | IFN-γ
|
IL-2
|
IL-6
|
TNF-α
|
IL-12 p70*
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| KC | C | KC | C | KC | C | KC | C | KC | C | |
| SAC | ≤3 | 1,104 | 37 | ≤7 | 156 | 10,000 | 1,138 | 13,568 | ≤5 | 32 |
| SEA | 241 | 1,146 | 1,208 | 2,472 | 156 | 600 | 11,295 | 10,633 | ≤5 | 441 |
| PMA + iono | 1,170 | 1,138 | 2,619 | 2,381 | 10,000 | 10,000 | 9,971 | 6,748 | ≤5 | ≤5 |
| Medium | ≤3 | ≤3 | ≤7 | ≤7 | ≤3 | ≤3 | ≤3 | 27 | ≤5 | ≤5 |
PBMCs from the patient (KC) or control (C) were incubated with SAC, SEA, or PMA plus ionomycin (iono) for 24 h. Cytokine yields (pg/ml) in the culture supernatants were measured by ELISA. Results are expressed as the mean of triplicate cultures. The deviation from the mean is always <10% of the mean value.
IL-12 determinations were performed in three separate experiments. A 48-h culture of IL-12 supernatant also was tested. The result of KC’s IL-12 at 48 h was ≤5. C IL-12 was 241 pg/ml.
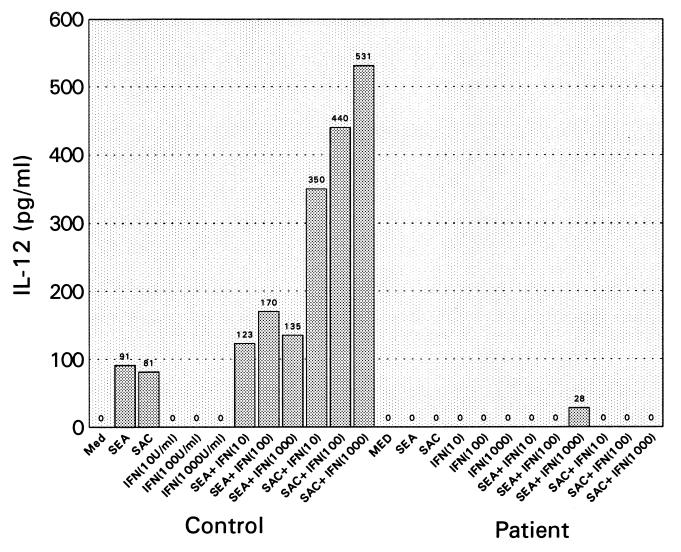
We subsequently tested IL-12 p70 production by ELISA from supernatants of the patient’s PBMCs after stimulation with SEA for 24 and 48 h, respectively. As shown in Table 4, the patient’s cells did not produce IL-12 p70 under these conditions, whereas supernatants of SEA-stimulated control PBMCs produced 441 pg/ml and 241 pg/ml of IL-12, respectively (see Table 4 legend). Because IFN-γ was reduced by using patient’s cells in SAC- as well as SEA-stimulated cells, we performed an additional experiment, whereby IFN-γ was added in vitro to both control and patient cells. As shown in Fig. 1, IL-12 production was observed by using control cells with SEA or SAC. When IFN-γ was added in varying concentrations to SEA- or SAC-stimulated control cells, IL-12 production was enhanced twofold with SEA-stimulated cells and sixfold with SAC-stimulated cells. By contrast, IL-12 production upon the addition of IFN-γ was not enhanced when the patient’s cells were treated in a manner identical with control cells.
Figure 1.
IL-12 p70 production in the presence of IFN-γ. PBMCs from the patient or control were incubated with SEA (10 ng/ml) or SAC (1:10,000, wt/vol) in the presence of IFN-γ at varying concentrations. The cultures were incubated for 24 h at 37°C, and the supernatants were then tested for IL-12 p70 production by ELISA.
The observations that the supernatants of SAC-stimulated PBMCs cannot produce bioactive IL-12 p70 are provocative, because the patient’s PBMCs show a high proliferative response to SAC.
Absence of p40 Subunit mRNA Expression in PBMCs of This Patient.
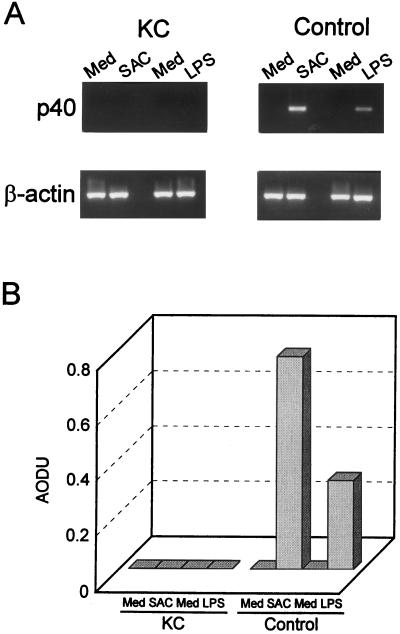
We further investigated whether the deficiency of IL-12 production of the patient is regulated at the mRNA level. In this experiment, we tested the mRNA expression of IL-12 p40 and p35 subunits after stimulation with SAC or LPS. As shown in Fig. 2A, stimulation with SAC or LPS resulted in a rapid increase in mRNA levels for the IL-12 p40 subunit in normal control cells. By contrast, neither SAC nor LPS induced IL-12 p40 mRNA expression in the patient’s PBMCs by reverse transcription–PCR. By scanning densitometric analyses, the values for SAC- or LPS-induced IL-12 p40 of the normal control cells based on the level of β-actin were 0.767 and 0.318 arbitrary optical density units, respectively (Fig. 2B), but IL-12 p40 expression by using the patient’s cells could not be demonstrated at all. Induction of IL-12 p35 subunit mRNA by SAC or LPS was not strong enough in either the patient’s cells or the control cells to permit the levels between the patient and control to be compared.
Figure 2.
(A) Expression of IL-12 p40 mRNA. PBMCs from the patient (KC) or from healthy controls were incubated with SAC or medium alone (experiment 1) or with LPS or medium alone (experiment 2) for 4 h. Next, total RNA was extracted, and reverse transcription–PCR was performed by using specific primers for human IL-12 p40 subunit or β-actin. The sizes of the PCR products for IL-12 p40 and β-actin are 465 bp and 510 bp, respectively. (B) Densitometric analysis of IL-12 p40 mRNA levels. The IL-12 p40 band densities were normalized to the corresponding β-actin densities and expressed as arbitrary optical density units (AODU). Results are representative of three separate experiments.
DISCUSSION
In the present study, we describe a child with recurrent infections whose PBMCs fail to produce IL-12; we also show reduced production of IFN-γ. In this context, it has been shown by previous investigators that a positive feedback mechanism between IL-12 and IFN-γ production exists (18–22). Furthermore, IL-12 p40 knockout mice are severely impaired in their in vivo LPS- or ex vivo Ag-induced production of IFN-γ but not in their production of IL-2, IL-4, or IL-10 (23). In addition, IL-12 p40 knockout mice previously transplanted with cardiac allografts produced low IFN-γ levels upon ex vivo restimulation with donor alloantigens (24). Other similarities have been shown in patients with familial disseminated Mycobacterium avium disease complex. Such patients, who produce low amounts of IFN-γ, also exhibit defective IL-12 production. The defective IL-12 production is attributed to an abnormal regulation of IL-12 production by monocytes (25). In the latter, IL-12 was markedly enhanced by the addition of IFN-γ to the stimulated cells (25). In our experiments, the addition of IFN-γ to cells of our patient made no difference in the SEA- or SAC-induced production of IL-12 when compared with control cells (Fig. 1).
mRNA expression of the IL-12 p40 subunit has been shown to be highly regulated and to be rapidly induced after stimulation of the producer cells. The p40 transcripts are present only in cell types that produce the biologically active IL-12 heterodimer, whereas the p35 subunit transcripts are expressed ubiquitously in almost all cell types, including cells that are not expressing the p40 mRNA and not producing IL-12 (26). In the light of these findings, the present results indicate that the deficiency of IL-12 p70 production by the patient’s cells is regulated at the IL-12 p40 mRNA level.
Our patient’s PBMCs exhibited normal proliferative responses to Ags as well as lectins. In this context, it is of interest that IL-12 p40 knockout mice display normal proliferative responses to Con A, anti-CD3, and IL-2 and have a normal distribution of CD4+ and CD8+ subsets of lymphocytes (23, 27). These investigators also showed that all of the parameters (i.e., hematology, serum chemistry, gross necroscopy, and histopathology) in the tissue of these mice were within normal limits (23, 27).
Despite the fact that proliferative responses of the patient’s PBMCs to SAC were significantly higher than those of controls, the patient’s PBMCs did not express IL-12 p40 mRNA upon stimulation with SAC; this finding indicates that SAC-induced growth responses and IL-12 p40 gene-activation mechanisms are under independent control, and that the cellular responses of the patient’s cells to SAC-induced growth signaling are intact.
Although SAC is a potent stimulator of B lymphocytes, normal B lymphocytes from healthy donors appear to be poor producers of IL-12 (26). Monocytes/macrophages are physiologically the main producers of IL-12 in vivo and in vitro (15, 26, 27). Both SAC and LPS are also potent stimulators of monocytes/macrophages. It has been shown that CD14, a monocyte/macrophage surface protein, is an essential receptor for LPS (28). However, the expression of CD14 in this patient’s PBMCs is normal as assessed by flow cytometric analysis (Table 2).
Taken together, these observations indicate that the patient may have an impairment in SAC-, LPS-, and/or SEA-activated signaling pathway(s) for IL-12 p40 gene expression in monocytes/macrophages. Another more attractive possibility is that the patient may have an abnormality of the IL-12 p40 gene. Molecular biological studies on the intracellular signaling defect as well as an analysis of the IL-12 p40 gene of the patient will be necessary to resolve these possibilities.
The present studies should contribute to a better understanding of the molecular mechanism(s) involved in the immunodeficiency state exhibited by this patient and also should focus the further elucidation of the causal basis of the decreased IL-12 production that has been repeatedly demonstrated in our patient.
Acknowledgments
We thank Michelle James-Yarish and Robert Follett, Jr. for technical assistance and Claudine Baird and Tazim Verjee for the preparation of this manuscript. This work was supported by grants from the U.S. Public Health Service Institute for Aging (AG05628–13), the Eleanor Naylor Dana Charitable Trust, and the Pediatric Cancer Foundation to Children’s Research Institute, All Children’s Hospital.
ABBREVIATIONS
- IL
interleukin
- PBMC
peripheral blood mononuclear cell
- SAC
Staphylococcus aureus Cowan strain 1
- LPS
lipopolysaccharide
- SEA
staphylococcal enterotoxin A
- TNF
tumor necrosis factor
- IFN
interferon
- Ag
antigen
- PMA
phorbol 12-myristate 13-acetate
References
- 1. DiSanto J P, Keever C A, Small T N, Nicols G L, O’Reilly R J, Flomenberg N. J Exp Med. 1990;171:1697–1704. doi: 10.1084/jem.171.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg K, Parkman R. N Engl J Med. 1990;322:1718–1723. doi: 10.1056/NEJM199006143222406. [DOI] [PubMed] [Google Scholar]
- 3.Chatila T, Castigli E, Pahwa R, Pahwa S, Chirmule N, Oyaizu N, Good R A, Geha R S. Proc Natl Acad Sci USA. 1990;87:10033–10037. doi: 10.1073/pnas.87.24.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 5.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J F, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova J L. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 6.Pierre-Audigier C, Jouanguy E, Lamhamedi S, Altare F, Rauzier J, Vincent V, Canioni D, Emile J F, Fischer A, Blanche S, Gaillard J L, Casanova J L. Clin Infect Dis. 1997;24:982–984. doi: 10.1093/clinids/24.5.982. [DOI] [PubMed] [Google Scholar]
- 7.Casanova J-L, Newport M, Fischer A, Levin M. In: Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. Ochs D H, Smith C I E, Puck J M, editors. New York: Oxford Univ. Press; 1998. , in press. [Google Scholar]
- 8.Dorman S E, Holland S M. J Clin Invest. 1998;101:2364–2369. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altare F, Durandy A, Lammas D, Emile J-F, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin R, et al. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 10.de Jong R, Altare F, Haagen IA, Elferink D G, Boer T, van Breda Vriesman P J, Kabel P J, Draaisma J M T, van Dissel J T, Kroon F P, et al. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern A S, Podlaski F J, Hulmes J D, Pan Y E, Quinn P M, Wolitzky A G, Familletti P C, Stremlo D L, Truitt T, Chizzonite R, Gately M K. Proc Natl Acad Sci USA. 1990;87:6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf S F, Temple P A, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick R M, et al. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 14.Gubler U, Chua A O, Schoenhaut D S, Dwyer C M, McComas W, Motyka R, Nabavi N, Wolitzky A G, Quinn P M, Familletti P C, Gately M K. Proc Natl Acad Sci USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chehimi J, Trinchieri G. J Clin Immunol. 1994;14:149–161. doi: 10.1007/BF01533364. [DOI] [PubMed] [Google Scholar]
- 16.Brunda M J. J Leukocyte Biol. 1994;55:280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 17.Biron C A, Gazzinelli R T. Curr Opin Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 18.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 19.McDyer J F, Goletz T J, Thomas E, June C H, Seder R A. J Immunol. 1998;160:1701–1707. [PubMed] [Google Scholar]
- 20.Kubin M, Chow J M, Trinchieri G. Blood. 1994;83:1847–1855. [PubMed] [Google Scholar]
- 21.Flesch I E, Hess J H, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann S H. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes M P, Wang J, Norcross M A. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 23.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 24.Piccotti J R, Chan S Y, Goodman R E, Magram J, Eichwald E J, Bishop D K. J Immunol. 1996;157:1951–1957. [PubMed] [Google Scholar]
- 25.Frucht D M, Holland S M. J Immunol. 1996;157:411–416. [PubMed] [Google Scholar]
- 26.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, et al. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamont A G, Adorini L. Immunol Today. 1996;17:214–217. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- 28.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]