Abstract
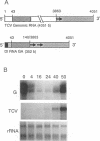
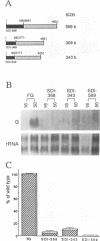
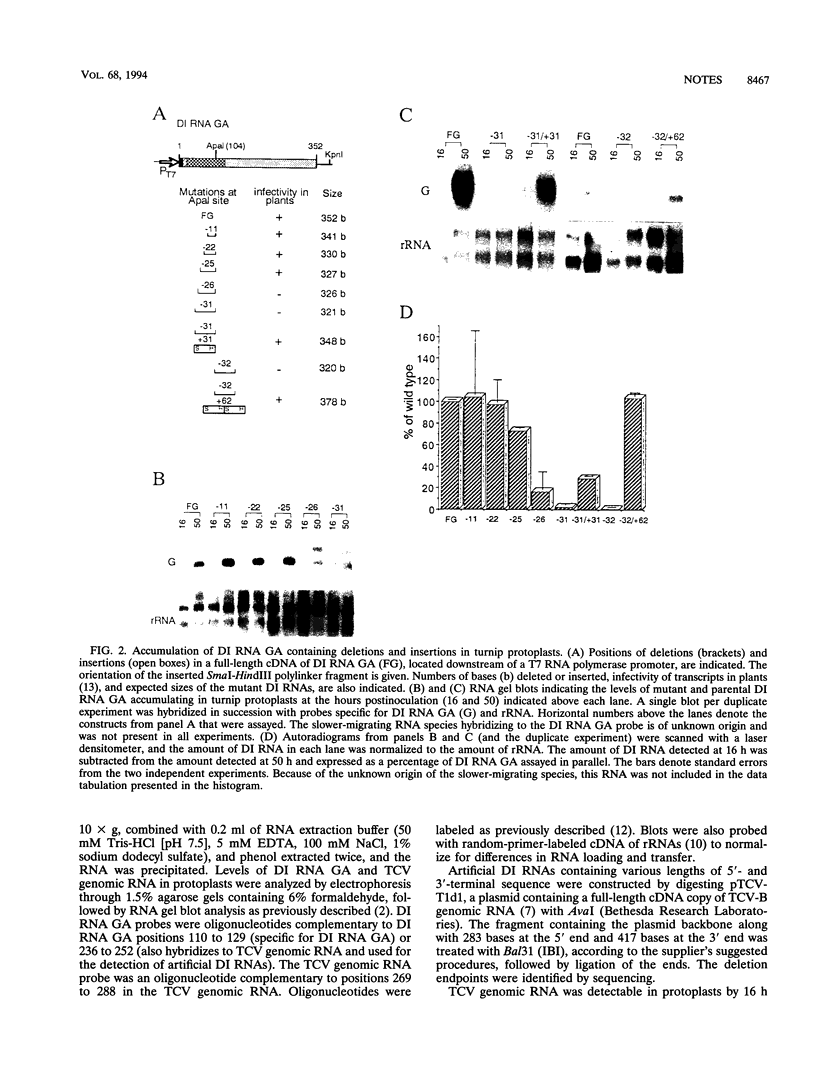
A turnip protoplast system has been used to study the effects of template size and sequence on the replication and/or stability of a small defective interfering (DI) RNA associated with turnip crinkle virus. Our results indicated that as little as a single base difference in the size of the molecule in some regions, rather than the specific sequence, affected the level of DI RNA accumulating in protoplasts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calain P., Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993 Aug;67(8):4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. D., Simon A. E. Recombination between plus and minus strands of turnip crinkle virus. Virology. 1994 Jun;201(2):419–423. doi: 10.1006/viro.1994.1312. [DOI] [PubMed] [Google Scholar]
- Fosmire J. A., Hwang K., Makino S. Identification and characterization of a coronavirus packaging signal. J Virol. 1992 Jun;66(6):3522–3530. doi: 10.1128/jvi.66.6.3522-3530.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton L. A., Carrington J. C., Morris T. J. Turnip crinkle virus infection from RNA synthesized in vitro. Virology. 1989 May;170(1):214–218. doi: 10.1016/0042-6822(89)90368-1. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Jeong Y. S., Makino S. Evidence for coronavirus discontinuous transcription. J Virol. 1994 Apr;68(4):2615–2623. doi: 10.1128/jvi.68.4.2615-2623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Weiss B. G., Tsiang M., Huang H., Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986 Jan 17;44(1):137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- Li X. H., Heaton L. A., Morris T. J., Simon A. E. Turnip crinkle virus defective interfering RNAs intensify viral symptoms and are generated de novo. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9173–9177. doi: 10.1073/pnas.86.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H., Simon A. E. In vivo accumulation of a turnip crinkle virus defective interfering RNA is affected by alterations in size and sequence. J Virol. 1991 Sep;65(9):4582–4590. doi: 10.1128/jvi.65.9.4582-4590.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Yokomori K., Lai M. M. Analysis of efficiently packaged defective interfering RNAs of murine coronavirus: localization of a possible RNA-packaging signal. J Virol. 1990 Dec;64(12):6045–6053. doi: 10.1128/jvi.64.12.6045-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re G. G., Kingsbury D. W. Paradoxical effects of Sendai virus DI RNA size on survival: inefficient envelopment of small nucleocapsids. Virology. 1988 Aug;165(2):331–337. doi: 10.1016/0042-6822(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Roux L., Simon A. E., Holland J. J. Effects of defective interfering viruses on virus replication and pathogenesis in vitro and in vivo. Adv Virus Res. 1991;40:181–211. doi: 10.1016/S0065-3527(08)60279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang M., Weiss B. G., Schlesinger S. Effects of 5'-terminal modifications on the biological activity of defective interfering RNAs of Sindbis virus. J Virol. 1988 Jan;62(1):47–53. doi: 10.1128/jvi.62.1.47-53.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Bancroft J. B., Mackie G. A. Coding capacity determines in vivo accumulation of a defective RNA of clover yellow mosaic virus. J Virol. 1992 May;66(5):3069–3076. doi: 10.1128/jvi.66.5.3069-3076.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., van der Most R. G., Spaan W. J. The fitness of defective interfering murine coronavirus DI-a and its derivatives is decreased by nonsense and frameshift mutations. J Virol. 1992 Oct;66(10):5898–5905. doi: 10.1128/jvi.66.10.5898-5905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most R. G., Bredenbeek P. J., Spaan W. J. A domain at the 3' end of the polymerase gene is essential for encapsidation of coronavirus defective interfering RNAs. J Virol. 1991 Jun;65(6):3219–3226. doi: 10.1128/jvi.65.6.3219-3226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most R. G., de Groot R. J., Spaan W. J. Subgenomic RNA synthesis directed by a synthetic defective interfering RNA of mouse hepatitis virus: a study of coronavirus transcription initiation. J Virol. 1994 Jun;68(6):3656–3666. doi: 10.1128/jvi.68.6.3656-3666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]