Abstract
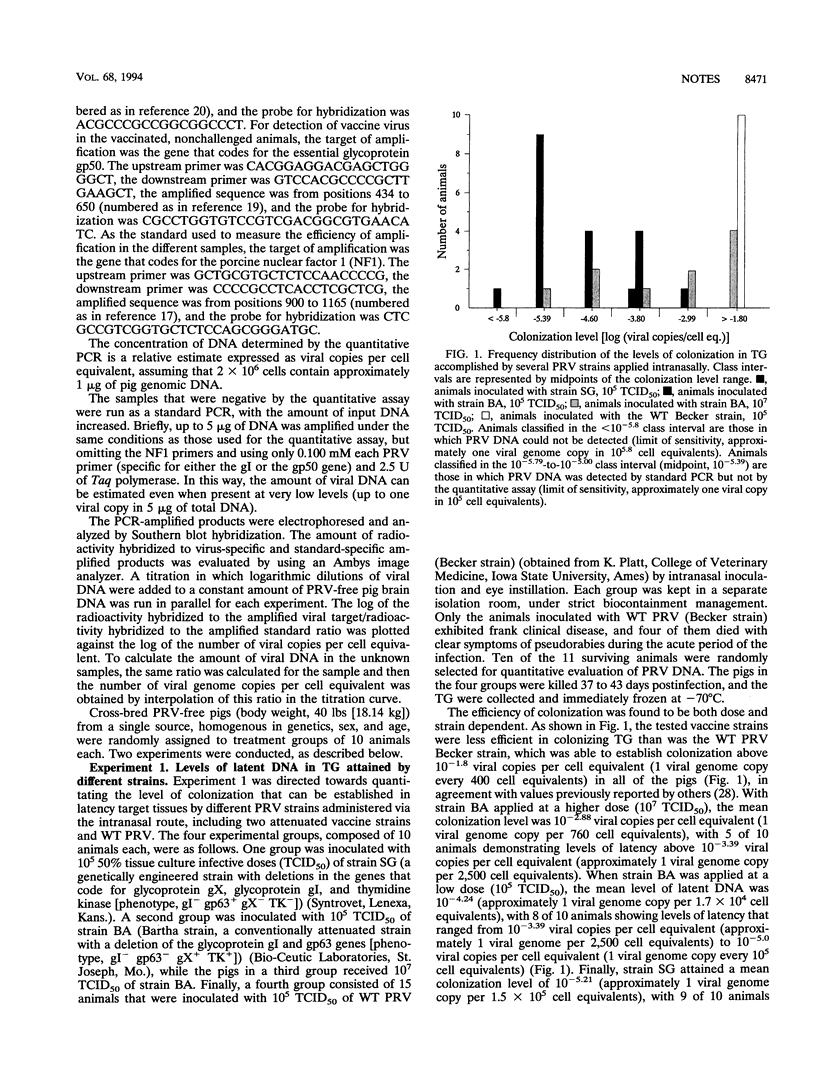
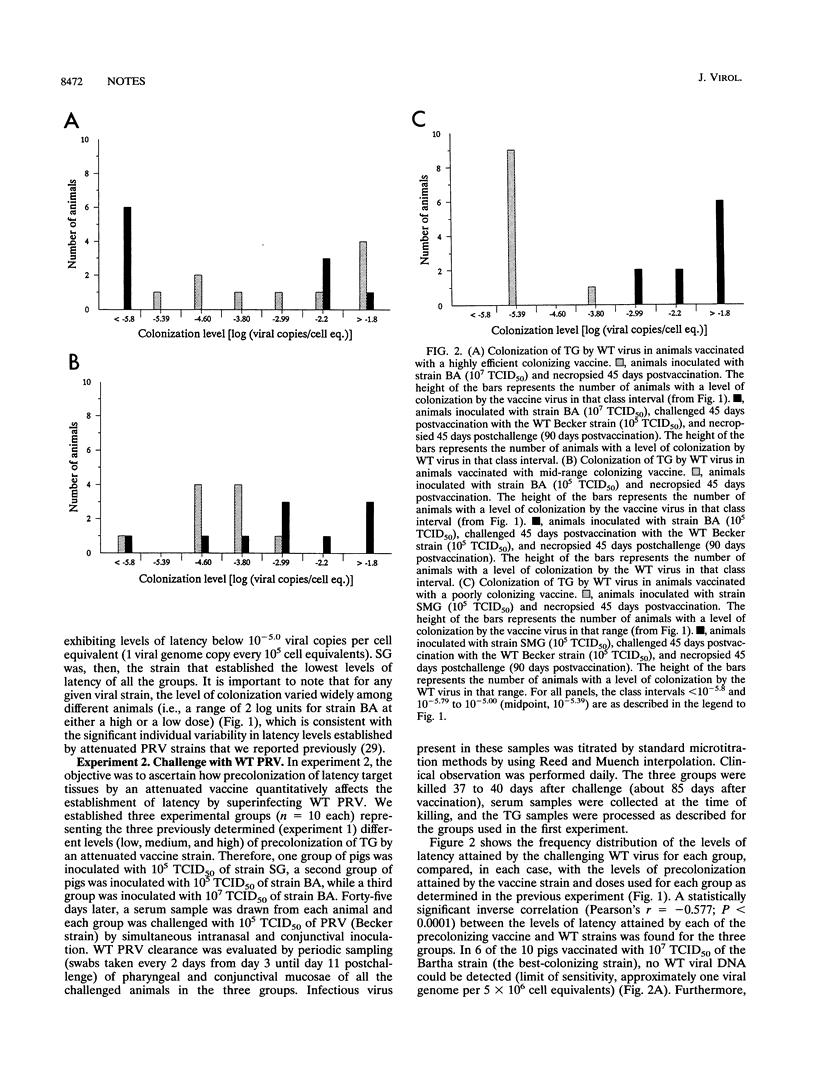
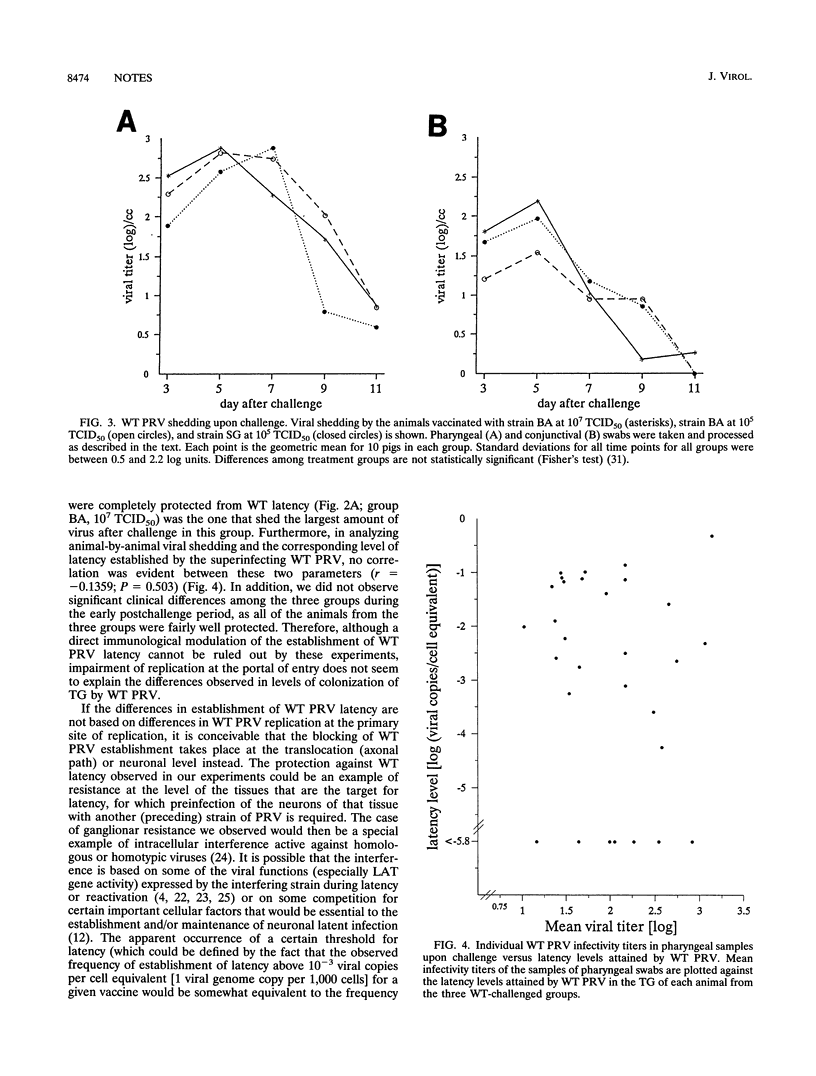
We compared the levels of latent pseudorabies virus (PRV) DNA in trigeminal ganglia (TG) of pigs after intranasal inoculation of different PRV strains by using quantitative DNA PCR. The extent of colonization attained in each case varied significantly according to the type of strain and inoculum dose, wild-type (WT) PRV being the most efficient strain in colonizing TG. When groups of pigs representing different levels of precolonization of TG with an attenuated PRV strain were challenged with WT PRV, it became evident that there is a statistically significant inverse correlation between the extent of precolonization attained by an attenuated PRV strain in TG and the level of establishment of latency by superinfecting WT PRV. The protection against WT PRV latency did not correlate with the extent of WT PRV replication at the portal of entry.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Card J. P., Whealy M. E., Robbins A. K., Enquist L. W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992 May;66(5):3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centifanto-Fitzgerald Y. M., Varnell E. D., Kaufman H. E. Initial herpes simplex virus type 1 infection prevents ganglionic superinfection by other strains. Infect Immun. 1982 Mar;35(3):1125–1132. doi: 10.1128/iai.35.3.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Valyi-Nagy T. Viral, neuronal and immune factors which may influence herpes simplex virus (HSV) latency and reactivation. Microb Pathog. 1993 Aug;15(2):83–91. doi: 10.1006/mpat.1993.1059. [DOI] [PubMed] [Google Scholar]
- Jacobson J. G., Ruffner K. L., Kosz-Vnenchak M., Hwang C. B., Wobbe K. K., Knipe D. M., Coen D. M. Herpes simplex virus thymidine kinase and specific stages of latency in murine trigeminal ganglia. J Virol. 1993 Nov;67(11):6903–6908. doi: 10.1128/jvi.67.11.6903-6908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A. K., Nash A. A., Wildy P. Pathogenesis of herpes simplex virus in B cell-suppressed mice: the relative roles of cell-mediated and humoral immunity. J Gen Virol. 1982 Jul;61(Pt 50):127–131. doi: 10.1099/0022-1317-61-1-127. [DOI] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., Brady E. Latent herpes simplex virus in ganglia of mice after primary infection and reinoculation at a distant site. Arch Virol. 1978;57(2):161–166. doi: 10.1007/BF01315677. [DOI] [PubMed] [Google Scholar]
- Klein R. J. The pathogenesis of acute, latent and recurrent herpes simplex virus infections. Arch Virol. 1982;72(3):143–168. doi: 10.1007/BF01348961. [DOI] [PubMed] [Google Scholar]
- Lewis M. E., Leung W. C., Jeffrey V. M., Warren K. G. Detection of multiple strains of latent herpes simplex virus type 1 within individual human hosts. J Virol. 1984 Oct;52(1):300–305. doi: 10.1128/jvi.52.1.300-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A., Dent C. L., Wheatley S. C., Beech M. N., Ninkina N. N., Wood J. N., Latchman D. S. The octamer-binding protein Oct-2 represses HSV immediate-early genes in cell lines derived from latently infectable sensory neurons. Neuron. 1991 Sep;7(3):381–390. doi: 10.1016/0896-6273(91)90290-g. [DOI] [PubMed] [Google Scholar]
- Margolis T. P., Sedarati F., Dobson A. T., Feldman L. T., Stevens J. G. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology. 1992 Jul;189(1):150–160. doi: 10.1016/0042-6822(92)90690-q. [DOI] [PubMed] [Google Scholar]
- Meignier B. Genetically engineered attenuated herpes simplex viruses. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S895–S897. doi: 10.1093/clind/13.supplement_11.s895. [DOI] [PubMed] [Google Scholar]
- Meignier B., Norrild B., Roizman B. Colonization of murine ganglia by a superinfecting strain of herpes simplex virus. Infect Immun. 1983 Aug;41(2):702–708. doi: 10.1128/iai.41.2.702-708.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisterernst M., Rogge L., Foeckler R., Karaghiosoff M., Winnacker E. L. Structural and functional organization of a porcine gene coding for nuclear factor I. Biochemistry. 1989 Oct 3;28(20):8191–8200. doi: 10.1021/bi00446a034. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C. Molecular biology of pseudorabies (Aujeszky's disease) virus. Comp Immunol Microbiol Infect Dis. 1991;14(2):151–163. doi: 10.1016/0147-9571(91)90128-z. [DOI] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Armentrout M. A., Marchioli C. C., Yancey R. J., Jr, Post L. E. DNA sequence of the gene for pseudorabies virus gp50, a glycoprotein without N-linked glycosylation. J Virol. 1986 Aug;59(2):216–223. doi: 10.1128/jvi.59.2.216-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Post L. E. Use of lambda gt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986 Oct;60(1):185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Khan A. Resistance of peripheral autonomic neurons to in vivo productive infection by herpes simplex virus mutants deficient in thymidine kinase activity. Infect Immun. 1981 Nov;34(2):571–580. doi: 10.1128/iai.34.2.571-580.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priola S. A., Gustafson D. P., Wagner E. K., Stevens J. G. A major portion of the latent pseudorabies virus genome is transcribed in trigeminal ganglia of pigs. J Virol. 1990 Oct;64(10):4755–4760. doi: 10.1128/jvi.64.10.4755-4760.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priola S. A., Stevens J. G. The 5' and 3' limits of transcription in the pseudorabies virus latency associated transcription unit. Virology. 1991 Jun;182(2):852–856. doi: 10.1016/0042-6822(91)90628-o. [DOI] [PubMed] [Google Scholar]
- Roizman B. Introduction: objectives of herpes simplex virus vaccines seen from a historical perspective. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S892–S894. doi: 10.1093/clind/13.supplement_11.s892. [DOI] [PubMed] [Google Scholar]
- Rziha H. J., Mettenleiter T. C., Ohlinger V., Wittmann G. Herpesvirus (pseudorabies virus) latency in swine: occurrence and physical state of viral DNA in neural tissues. Virology. 1986 Dec;155(2):600–613. doi: 10.1016/0042-6822(86)90220-5. [DOI] [PubMed] [Google Scholar]
- Simmons A., Nash A. A. Effect of B cell suppression on primary infection and reinfection of mice with herpes simplex virus. J Infect Dis. 1987 Apr;155(4):649–654. doi: 10.1093/infdis/155.4.649. [DOI] [PubMed] [Google Scholar]
- Thomas E., Lycke E., Vahlne A. Retrieval of latent herpes simplex virus type 1 genetic information from murine trigeminal ganglia by superinfection with heterotypic virus in vivo. J Gen Virol. 1985 Aug;66(Pt 8):1763–1770. doi: 10.1099/0022-1317-66-8-1763. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Koprowski H., Lonsdale D. M., Brown S. M., Subak-Sharpe J. H. The polypeptide and the DNA restriction enzyme profiles of spontaneous isolates of herpes simplex virus type 1 from explants of human trigeminal, superior cervical and vagus ganglia. J Gen Virol. 1979 Apr;43(1):151–171. doi: 10.1099/0022-1317-43-1-151. [DOI] [PubMed] [Google Scholar]
- Whealy M. E., Card J. P., Robbins A. K., Dubin J. R., Rziha H. J., Enquist L. W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993 Jul;67(7):3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J. G., Osorio F. A. Investigation of sites of pseudorabies virus latency, using polymerase chain reaction. Am J Vet Res. 1991 Nov;52(11):1799–1803. [PubMed] [Google Scholar]
- Whetstone C. A., Miller J. M. Two different strains of an alphaherpesvirus can establish latency in the same tissue of the host animal: evidence from bovine herpesvirus 1. Arch Virol. 1989;107(1-2):27–34. doi: 10.1007/BF01313875. [DOI] [PubMed] [Google Scholar]
- van Oirschot J. T., Gielkens A. L., Moormann R. J., Berns A. J. Marker vaccines, virus protein-specific antibody assays and the control of Aujeszky's disease. Vet Microbiol. 1990 Jun;23(1-4):85–101. doi: 10.1016/0378-1135(90)90139-m. [DOI] [PubMed] [Google Scholar]



