Abstract
Objective
To understand estrogen regulation of proton (H+) secretion by human vaginal-ectocervical epithelial cells and the mechanisms involved.
Design
Primary-tertiary cultures of human normal vaginal-ectocervical epithelial cells were generated from surgical specimens of premenopausal women (aged 37-46 years) and of postmenopausal women (aged 53-65 years). Cells were grown on filters, and measurements were made of changes in extracellular pH (pHo) in the contraluminal (CL) and luminal (L) solutions 30 minutes after shifting cells to basic salt solution.
Results
Upon shifting cells to basic salt solution, CL-pHo decreased from 7.4 to 7.25, and was not affected by removal of intracellular estrogens or treatment with estradiol. L-pHo decreased from 7.4 to 7.05 in cells of premenopausal women, and from 7.4 to 7.20 in cells of postmenopausal women. Removal of intracellular estrogens attenuated the decrease in L-pHo in cells of premenopausal women (only to 7.20). In cells of premenopausal women stripped of estrogens, treatment with 10 nM 17β-estradiol restored the decrease in L-pHo. In estrogen-stripped cells of postmenopausal women, treatment with estradiol augmented luminal acidification but to a lesser degree than in cells of premenopausal women (L-pHo of 7.15 vs 7.05). In cells of pre- and postmenopausal women, the addition in the L solution of bafilomycin-A1, a specific inhibitor of the vacuolar-H+-ATPase (V-H+-ATPase), blocked the decrease in L-pHo.
Conclusions
Human vaginal-ectocervical epithelial cells acidify constitutively their luminal solution, and the effect is mediated by active H+ secretion by V-H+-ATPase expressed predominantly in the apical cell membrane. Estrogen deprivation attenuates, and treatment with 17β-estradiol augments, active H+ secretion. Finally, cells of postmenopausal women actively secrete H+ via apically located V-H+-ATPase, but the effect is lesser, and estrogen failed to augment active H+ secretion, as in cells of premenopausal women. These data suggest that in addition to hypo-estrogenism, other factors of the aging process affect the capacity of vaginal-ectocervical cells to secrete acid.
Keywords: pH, Vaginal, Cervical, Proton, Apical, Luminal, Epithelium
The main function of the acidic environment of the vaginal lumen is to control infections of the lower genital tract. The vaginal acid-base environment is tightly regulated, and during premenopausal years vaginal-fluid pH ranges from 4.5 to 5.5 with mild alkalinization to about 6.0 before ovulation.
Despite its important role, relatively little is known about mechanisms that regulate vaginal-fluid pH in women. Until recently, it was believed that the acidic milieu is determined by cohabituating Doderlein lactobacilli.1 We recently presented data that refute the Doderlein theory by showing that human normal vaginal-ectocervical epithelial cells grown on filters under sterile conditions acidify their luminal solution.2 The effect was estrogen-dependent, and could be blocked by low micromolar concentrations of bafilomycin-A1, indicating active proton (H+) secretion by V-type H+-ATPase3 that is located in the apical membrane (Fig. 1). In this regard, vaginal epithelial cells resemble other H+ secreting cells that regulate acid secretion into the lumen.4-9
FIG. 1.
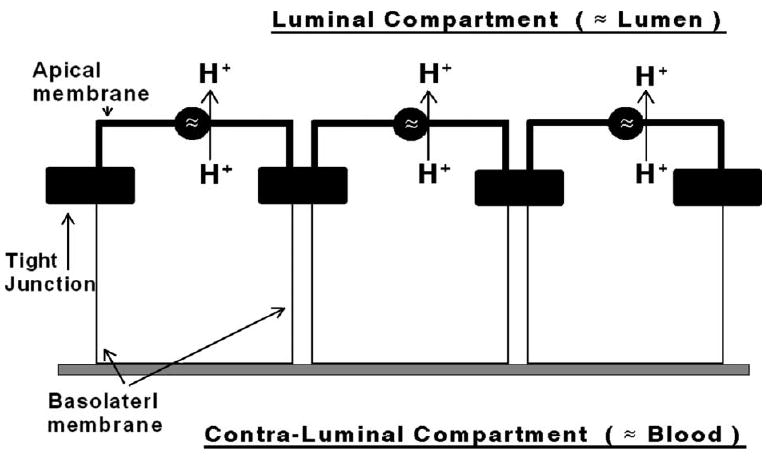
Hypothesis of the present study. Vaginal-ectocervical epithelial cells acidify the lumen by active extrusion of protons via proton transport mechanisms located in the apical cell membrane. Tight junctions (filled horizontal bars) separate the plasma membranes into apical (bold lines) and basolateral domains, facing respectively the luminal (“lumen”) and contraluminal (“blood”) compartments.
After menopause, vaginal-fluid pH increases to 6.5 to 7.0.10 Elevated vaginal-fluid pH has been associated with urinary tract infections,11 vaginal atrophy,12 and dyspareunia.13 Treatment with estrogen can acidify the luminal vaginal pH to 5.5 to 6.5,10 suggesting that alkalinization of the vaginal-fluid pH in postmenopausal women is the result of hypoestrogenism. To test this hypothesis more directly, we used our novel culture system of filter generated vaginal-ectocervical cultured epithelia to determine effects of estrogen on luminal proton secretion in cells of postmenopausal women. The results indicate that treatment with estrogen increases active proton secretion by cells of postmenopausal women. However, luminal pH remained elevated compared with that of cells of premenopausal women, suggesting that in addition to hypoestrogenism, other factors of the aging process affect the capacity of vaginal-ectocervical cells to secrete acid.
METHODS
Cell cultures
The experiments used primary-tertiary cultures of human vaginal-ectocervical epithelial (hECE) cells generated from tissues of the vagina-ectocervix and cultured as described elsewhere.14-19 The hECE cells were previously characterized as phenotypically resembling the stratified squamous vaginal-ectocervical epithelial cells.14-17 Tissues were collected from nine premenopausal women aged 37 to 46 years and from eight postmenopausal women aged 53 to 65 years. None of the women was treated with steroid sex hormones for at least 3 months. Menopause was defined as amenorrhea for at least 1 year, or amenorrhea of at least 6 months plus climacteric symptoms and plasma levels of follicle-stimulating hormone above 25 mIU/mL.
Determinations of changes in extracellular pH (pHo)
The method of determining changes in extracellular pH (pHo) was recently described.2 The hECE cells were plated on collagen-coated Anocell filters, either on the upper surface of the filter (for determinations of luminal pHo) or on the bottom surface of the filter (for determinations of the contraluminal pHo). In the latter case, after cells attached onto the filters, filters were flipped vertically to establish the filter’s upper compartment as housing the contraluminal solution, and the compartment in which the filter was bathed as housing the luminal solution. Plates containing filters were placed in a tissue culture incubator (37°C), and pHo changes in the luminal and contraluminal solutions were determined using AMANI-1000 microcombination pH electrodes (Harvard Apparatus, Holliston, MA, obtained through Genomics Solutions, Ann Harbor, MI) as described elsewhere.2 For experiments, the luminal and contraluminal solutions were replaced with fresh warmed (37°C) basic salt solution containing (in mM) NaCl (140), KC1 (5), MgC12 (1), CaCl2 (1), glucose (10), and 0.1% bovine serum albumin, pH 7.4. Determinations of changes in pHo were made at time 0, and at 5-minute intervals thereafter for up to 30 minutes.
Deprivation of estrogens
To remove intracellular estrogens cells were grown in steroid-free medium for 3 days as described previously.20 To replenish estrogen, cells shifted to steroid-free medium were treated with 17β-estradiol for 2 days before experiments. This low concentration, which is in the physiological range for the woman, is near maximal for increasing vaginal-ectocervical transepithelial paracellular permeability.20
Statistical analysis of the data
Data are presented as means (± SD) and significance of differences among means was estimated by Student’s t test. Trends were calculated using GB-STAT V5.3 (Dynamic Microsystems Inc., 1995, Silver Spring, MD) and analyzed with analysis of variance.
Chemicals
Chemicals were obtained from Sigma Chemical (St. Louis, MO). Stock chemicals and drug solutions were titrated to pH 7.4 prior to cell treatments, and were administered from Ã- 1,000 stocks.
RESULTS
hECE cells acidify the luminal solution
Upon shifting cells from regular culture medium to basic salt solution (pH 7.4), contraluminal pHo decreased in a time-related manner after 30 minutes to about 7.25, and the effect was similar in cultures of cells of premenopausal and postmenopausal women (Fig. 2A, P < 0.01 in both cases). Under the same experimental conditions, luminal pHo of cells of premenopausal women, decreased to 7.05 (Fig. 2B, P < 0.01), and luminal pHo of cells of postmenopausal women to 7.20 (Fig. 2B, P < 0.01). Results of five experiments are summarized in Fig. 2C, showing a decrease in luminal pHo to 7.03 ± 0.03 in cells of premenopausal women, and to 7.19 ± 0.02 in cells of postmenopausal women. In both cases, the decrease in luminal pHo was greater than the decrease in contraluminal pHo (Fig. 2C, P < 0.03-0.01). These data indicate that hECE cells acidify the luminal solution to a greater degree than the contraluminal solution, but the effect is more pronounced in cells of premenopausal women.
FIG. 2.
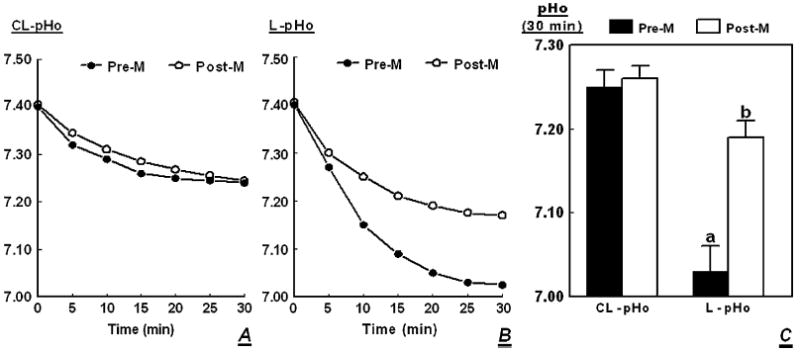
Effects of menopausal status on changes in pHo. The hECE cultures were generated from tissues of premenopausal (Pre-M) and postmenopausal (Post-M) women. At time t = 0, cultures were shifted to basic salt solution and changes in extracellular pH (pHo) were determined in the contraluminal (CL-pHo, A) and luminal (L-pHo, B) compartments as described in Methods. A and B show single experiments. C Means (± SD) of five experiments of changes in CL-pHo and L-pHo. a and b: P < 0.01 compared with CL-pHo.
Effects of estrogen on proton secretion
Incubation of hECE cells of pre- and postmenopausal women in steroid-free medium (SFM), and treatment with 10 nM 17β-estradiol estradiol (+E2) had no significant effect on contraluminal pHo, which decreased to 7.25 upon shifting cells to basic salt solution (Fig. 3A; compare with Fig. 2C, P > 0.1).
FIG. 3.
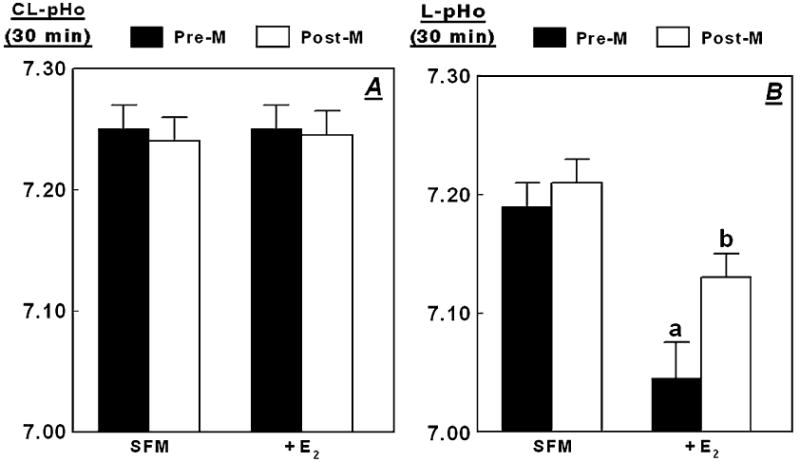
Effects of estrogen on changes in pHo. The hECE cells generated from tissues of premenopausal (Pre-M) and postmenopausal (Post-M) women were plated on filters and shifted to steroid-free medium for 1 day, and then maintained in the same medium for 2 additional days in the absence (SFM) or presence of 10 nM 17β-estradiol (added to both the luminal and contraluminal solutions) (+E2). For assays, cultures were shifted to basic salt solution in the continued absence or presence of 17β-estradiol. Shown are means (± SD) of three to five repeats per point of changes in pHo in the contraluminal (CL-pHo, A) or luminal compartments (L-pHo, B) 30 minutes after shifting cells to basic salt solution. In B, a and b: P < 0.01 compared with SFM.
Incubation of hECE cells of premenopausal women in SFM inhibited acidification of the luminal compartment (luminal pHo of 7.19 ± 0.02, Fig. 3B) compared with cells grown in regular medium (7.03 ± 0.02, Fig 2C, P < 0.01). These data indicate that deprivation of estrogens attenuates the constitutive active H+ secretion through the apical plasma membrane. Incubation of hECE cells of postmenopausal women in SFM resulted in acidification of the luminal pHo to 7.20 ± 0.02 (Fig. 3B), which was similar to that in cells of postmenopausal women grown in regular medium (7.19 ± 0.02, Fig. 2C, P > 0.1).
Treatment with 17β-estradiol of cells of premenopausal women grown in SFM augmented acidification of the luminal compartment (luminal pHo of 7.04 ± 0.03 compared to pHo of 7.19 ± 0.02 (Fig. 3B). Treatment with estradiol of cells of postmenopausal women grown in SFM also augmented acidification of the luminal compartment, but to a lesser degree (luminal pHo of 7.13 ± 0.02 compared with 7.20 ± 0.02, Fig. 3B, P < 0.01). These data indicate that treatment with estrogen augments H+ secretion through the apical plasma membrane, but the effect is lesser in cells of postmenopausal women compared to cells of premenopausal women.
Mechanism of the luminal acidification in cells of postmenopausal women
In hECE cells of premenopausal women, acidification of the luminal compartment is probably the result of active H+ secretion mediated by apically located vacuolar-type H+-ATPase (V-H+-ATPase).2 The objective of the next experiment was to determine to what degree the estrogen-induced luminal H+ secretion in cells of postmenopausal women (Fig. 3B) is also mediated by V-H+-ATPase. To test the hypothesis, hECE cells grown in SFM and treated with 10 nM 17β-estradiol were co-treated with 1 μ4M bafilomycin A1. Bafilomycin-A1 is a specific inhibitor of vacuolar-type H+-ATPases21,22 that inhibits the ATPase directly by binding to the transmembrane V0 subunit c of the ATPase.23,24
In cells of premenopausal women grown in SFM and treated with 10 nM 17β-estradiol, levels of luminal pHo 30 minutes after shifting cells to basic salt solution were 7.05 ± 0.02, compared with 7.15 ± 0.02 in cells of postmenopausal women (Fig. 4, empty bars, P < 0.01). These results confirm the data in Fig. 3, A and B. In both types of cells, co-treatment with bafilomycin A1 inhibited luminal acidification (Fig. 4, filled bars). Contraluminal administration of bafilomycin A1 attenuated luminal acidification slightly, probably because the drug leaked through the paracellular space to the luminal solution. In contrast, luminal administration of bafilomycin A1 blocked luminal acidification to pHo level of 7.25 ± 0.02 (Fig. 4), which was similar to the contraluminal pHo (compare with Figs. 2, A and C). These results suggest that in cells of premenopausal and postmenopausal women, the bafilomycin A1-sensitive H+-secreting mechanism (presumably V-H+-ATPase) is located on the apical side of the plasma membrane.
FIG. 4.
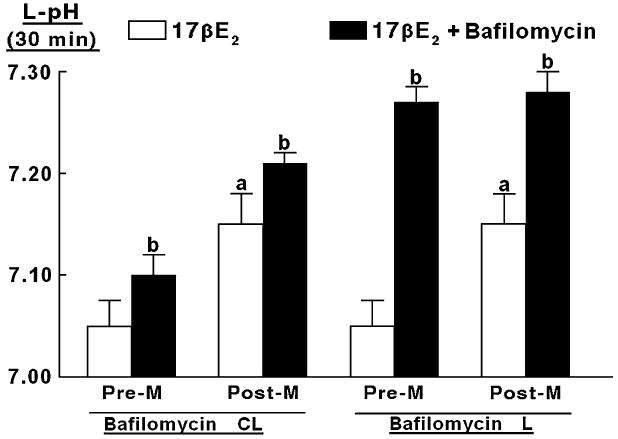
Modulation of estrogen-decrease in luminal pHo (L-pHo) by bafilomycin-A1. The hECE cells generated from tissues of premenopausal (Pre-M) and postmenopausal (Post-M) women were grown in steroid-free medium plus 10 nM 17β-estradiol (17βE2). Sixty minutes before assays, bafilomycin-A1 (1 μM) was added to the contraluminal (Bafilomycin-CL) or the luminal (Bafilomycin-L) solutions. Shown are means (± SD) of three to five repeats per point of L-pHo, determined 30 minutes after shifting cells to basic salt solution. a: P < 0.02 (Post-M vs Pre-M). b: P < 0.05-0.01 (17βE2 vs 17βE2 + bafilomycin).
Collectively, the results in Fig. 4 indicate that in cells of premenopausal and postmenopausal women net luminal H+ secretion is bafilomycin A1 sensitive, and therefore the estrogen-dependent component of luminal acidification in both types of cells is probably the apically located V-H+-ATPase mechanism.
DISCUSSION
The present results confirmed that human vaginal-ectocervical cells express estrogen-dependent, bafilomycin-A1-sensitive proton-secreting mechanism, which acidifies constitutively the luminal solution. The new findings of the study were that cells of postmenopausal women have a decreased capacity to acidify their luminal solution compared with premenopausal cells, and that treatment with estrogen can restore this function only in part.
The study used an experimental model that mimics the vaginal-ectocervical epithelium in vivo. The confluent cultures of cells could well separate the luminal and contraluminal solutions, representing respectively the “lumen” and “blood.” The data show that 30 minutes after shifting cells to basic salt solution (pH 7.4), cells acidify both the contraluminal and luminal solutions. Acidification of the contraluminal solution is most probably the result of proton secretion via the Na+/H+ exchanger,25,26 or of extrusion of organic acids to equilibrate the acidic intracellular pH (about 7.25, G.I. Gorodeski, unpublished results, 2003) with the extracellular solution. This process was not affected by estrogen status of the cells or by treatment with bafilomycin-A1, and is considered passive acid secretion.
Acidification of the luminal solution, however, was active, against the H+ electrochemical gradient. In hECE cells, bafilomycin-A1-sensitive luminal H+ secretion occurs constitutively, and is regulated by estrogen. Lack of estrogen inhibits H+ secretion, and in cells of premenopausal women treatment with physiological levels of 17β-estradiol restores H+ secretion (Gorodeski et al2, and present results). In cells of premenopausal women, the estrogen effect was dose-dependent (EC50 ≈ 1 nM) and could be blocked by co-treatment with ICI-182780,2 suggesting involvement of the classical nuclear estrogen receptor mechanism.
Less is known about estrogen-regulation of H+ secretion in hECE cells of postmenopausal women, and in particular of mechanisms that cause the lesser acidification of the luminal solution compared with cells of premenopausal women. One possible explanation is estrogen status of the cells. Primary-tertiary cultures of hECE cells retain phenotypic characteristics of the native epithelium, including hormonal status,20,27,28 and the attenuated luminal acidification by cells of postmenopausal women could reflect hypoestrogenic state of the cells. This speculation is supported by the finding that removal of intracellular estrogens decreased luminal acidification further, suggesting that even the low concentration of estrogens present in the culture medium, or the presence of agents with estrogenic activity such as phenol red,29 suffice to upregulate the proton extrusion mechanism.
CONCLUSION
Treatment of cells of postmenopausal women with 17β-estradiol increased H+ secretion, but luminal acidification did not reach the low pHo as in cells of premenopausal women. These data indicate that in addition to hypoestrogenism, other factors of the aging process affect the capacity of vaginal-ectocervical cells to secrete acid. More studies are needed to elucidate what aging-related mechanisms impair estrogen-modulation of luminal acidification by vaginal-ectocervical epithelial cell of postmenopausal women.
Supplementary Material
Acknowledgments
The technical support of Kimberley Frieden, Brian De-Santis, and Dipika Pal is acknowledged.
The study was supported by National Institute of Health Grants HD29924 and AG15955 (GIG), and by the Tissue Procurement Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland (P30 CA43703). Discarded tissues were collected according to IRB protocol 03-90-TG.
References
- 1.Weinstien L, Howard JH. The effect of estrogenic hormone on the H-ion concentration and the bacterial content of the human vagina with special reference to the Doderlein bacillus. Am J Obstet Gynecol. 1939;37:668–670. [Google Scholar]
- 2.Gorodeski GI, Hopfer U, Liu CC, Margles E. Estrogen acidifies vaginal pH by upregulation of proton secretion via the apical membrane of vaginal-ectocervical epithelial cells. Endocrinology. 2005;146:816–824. doi: 10.1210/en.2004-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishi T, Forgac M. The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 4.Sachs G. Physiology of the parietal cell and therapeutic implications. Pharmacotherapy. 2003;23:68S–73S. doi: 10.1592/phco.23.13.68s.31931. [DOI] [PubMed] [Google Scholar]
- 5.Li YP, Chen W, Liang Y, Li E, Stashenko P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet. 1999;23:447–451. doi: 10.1038/70563. [DOI] [PubMed] [Google Scholar]
- 6.Frattini A, Orchard PJ, Sobacchi C, et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 7.Brown D, Breton S. V-ATPase dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens. J Exp Biol. 2000;203:137–145. doi: 10.1242/jeb.203.1.137. [DOI] [PubMed] [Google Scholar]
- 8.Brisseau GF, Grinstein S, Hackam DJ, et al. IL-1 increases V-ATPase activity in murine peritoneal macrophages. J Biol Chem. 1996;271:2005–2011. doi: 10.1074/jbc.271.4.2005. [DOI] [PubMed] [Google Scholar]
- 9.Wieczorek H, Grber G, Harvey WR, Huss M, Merzendorfer H, Zeiske W. Structure and regulation of the insect plasma membrane V-ATPase. J Exp Biol. 2000;203:127–135. doi: 10.1242/jeb.203.1.127. [DOI] [PubMed] [Google Scholar]
- 10.Moller BR, Kaspersen P. The acidity of the vagina. In: Horowitz B, Mare PA, editors. Vaginitis and Vaginosis. New York, NY: Wiley-Liss; 1991. pp. 63–67. [Google Scholar]
- 11.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–756. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 12.Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci. 1997;314:228–231. doi: 10.1097/00000441-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Berman JR, Berman LA, Werbin TJ, Flaherty EE, Leahy NM, Goldstein I. Clinical evaluation of female sexual function: effects of age and estrogen status on subjective and physiologic sexual responses. Int J Impot Res. 1999;11:S31–S38. doi: 10.1038/sj.ijir.3900468. [DOI] [PubMed] [Google Scholar]
- 14.Gorodeski GI, Eckert RL, Utian WH, Rorke EA. Maintenance of in vivo-like keratin expression, sex steroid responsiveness and estrogen receptor expression in cultured human ectocervical epithelial cells. Endocrinology. 1990;126:399–406. doi: 10.1210/endo-126-1-399. [DOI] [PubMed] [Google Scholar]
- 15.Gorodeski GI, Romero MF, Hopfer U, Rorke E, Utian WH, Eckert RL. Human uterine cervical epithelial cells grown on permeable support - a new model for the study of differentiation and transepithelial transport. Differentiation. 1994;56:107–118. doi: 10.1046/j.1432-0436.1994.56120107.x. [DOI] [PubMed] [Google Scholar]
- 16.Gorodeski GI. The cultured human cervical epithelium: a new model for studying transepithelial paracellular transport. J Soc Gynecol Invest. 1996;3:267–280. doi: 10.1016/s1071-5576(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 17.Gorodeski GI, Pal D. Involvement of estrogen receptors a and b in the regulation of cervical permeability. Am J Physiol. 2000;278:C689–C696. doi: 10.1152/ajpcell.2000.278.4.C689. [DOI] [PubMed] [Google Scholar]
- 18.Gorodeski GI, Merlin D, De Santis BJ, et al. Characterization of paracellular permeability in cultured human cervical epithelium: regulation by extracellular ATP. J Soc Gynecol Invest. 1994;1:225–233. doi: 10.1177/107155769400100309. [DOI] [PubMed] [Google Scholar]
- 19.Gorodeski GI, Peterson D, De Santis BJ, Hopfer U. Nucleotide-receptor mediated decrease of tight-junctional permeability in cultured human cervical epithelium. Am J Physiol. 1996;270:C1715–C1725. doi: 10.1152/ajpcell.1996.270.6.C1715. [DOI] [PubMed] [Google Scholar]
- 20.Gorodeski GI. Estrogen increases the permeability of the cultured human cervical epithelium by modulating cell deformability. Am J Physiol. 1998;275:C888–C899. doi: 10.1152/ajpcell.1998.275.3.C888. [DOI] [PubMed] [Google Scholar]
- 21.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7996. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beutler JA, McKee TC. Novel marine and microbial natural product inhibitors of vacuolar ATPase. Curr Med Chem. 2003;10:787–796. doi: 10.2174/0929867033457827. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Feng Y, Forgac M. Proton conduction and bafilomycin binding by the V0 domain of the coated vesicle V-ATPase. J Biol Chem. 1994;269:23518–23523. [PubMed] [Google Scholar]
- 24.Bowman EJ, Graham LA, Stevens TH, Bowman BJ. The bafilomycin/concanamycin binding site in subunit c of the V-ATPases from Neurospora Crassa and Saccharomyces cerevisiae. J Biol Chem. 2004;279:33131–33138. doi: 10.1074/jbc.M404638200. [DOI] [PubMed] [Google Scholar]
- 25.Boron WF. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72:1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- 26.Aronson PS. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- 27.Gorodeski GI. Vaginal-cervical epithelial permeability decreases after menopause. Fertil Steril. 2001;76:753–761. doi: 10.1016/s0015-0282(01)02377-9. [DOI] [PubMed] [Google Scholar]
- 28.Gorodeski GI. Estrogen biphasic regulation of paracellular permeability of cultured human vaginal-cervical epithelia. J Clin Endocrinol Metab. 2001;86:4233–4243. doi: 10.1210/jcem.86.9.7817. [DOI] [PubMed] [Google Scholar]
- 29.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture medium is a weak estrogen: implication concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83:2496–2501. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


