Abstract
Previous studies have suggested neural disruption and reorganization in adult marijuana users. However, it remains unclear whether these effects persist in adolescents after 28 days of abstinence and, if they do, what Performance × Brain Response interactions occur. Adolescent marijuana users (n = 17) and controls (n = 17) aged 16–18 years were recruited from local schools. Functional magnetic resonance imaging data were collected after 28 days’ monitored abstinence as participants performed a spatial working memory task. Marijuana users show Performance × Brain Response interactions in the bilateral temporal lobes, left anterior cingulate, left parahippocampal gyrus, and right thalamus (clusters ≥ 1358 μl; p <.05), although groups do not differ on behavioral measures of task performance. Marijuana users show differences in brain response to a spatial working memory task despite adequate performance, suggesting a different approach to the task via altered neural pathways.
Keywords: marijuana, cannabis, adolescence, spatial working memory, functional magnetic resonance imaging
Marijuana is consistently the most widely used illicit drug among adolescents (Substance Abuse and Mental Health Services Administration, 2006). Forty-four percent of 12th-graders have used marijuana in their lifetime, 20% used in the past month, and 5% use daily (Johnston, O’Malley, Bachman & Schulenberg, 2006), representing a large increase from the 16% of eighth graders who have tried marijuana. Furthermore, 40% of high school students who used marijuana in the past year met criteria for marijuana abuse or dependence (Chen, Sheth, Elliot, & Eagerm, 2004). Marijuana use in adolescence causes significant concern because it may impact the brain, which is still developing throughout adolescence. Though overall brain size stabilizes around age five years (Durston et al., 2001), important progressive and regressive developmental processes continue throughout adolescence, including myelination (Giedd et al., 1999; Jernigan & Gamst, 2005), synaptic refinement (Huttenlocher & Dabholkar, 1997), reductions of gray matter volumes (Giedd et al., 1999; Gogtay et al., 2004; Sowell, Trauner, Gamst, & Jernigan, 2002), and improved cognitive and functional efficiency (Casey, Giedd, & Thomas, 2000; Durston et al., 2006). It is unclear how heavy marijuana use at this time could influence neural development. The long-term effects of marijuana have not yet been determined but could potentially have major implications on social, academic, and occupational functioning.
Although a good deal of research has been done on the effects of marijuana in chronic adult users, very little is known about adolescent users. Studies have shown that chronic marijuana has an influence on the neuropsychological performance of adults within a week of use. Specifically differences have been found in attention and executive functioning (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Pope, & Yurgelun-Todd, 1996), memory (Pope, Jacobs, Mialet, Yurgelun-Todd, & Gruber, 1997; Solowij et al., 2002), and psychomotor speed and manual dexterity (Croft, Mackay, Mills, & Gruzelier, 2001). One study demonstrated verbal learning deficits among marijuana users compared to controls 0, 1, and 7 days following use, but these impairments subsided after a 28-day abstinence period (Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001). However, others have identified impairments in memory, executive functioning, psychomotor speed, and manual dexterity compared to published norms after 28 days of verified abstinence (Bolla et al. 2002). Furthermore, adults who began use early in adolescence (before age 17 years) demonstrated greater decrements on verbal IQ after a 28-day abstinence period than those who began late in adolescence (after age 17 years) and nonusing controls, suggesting an adolescent vulnerability (Pope et al., 2003).
Due to its high safety profile and good spatial resolution, functional magnetic resonance imaging (fMRI) has become a powerful method for visualizing neural activation. Research on adult marijuana users has shown alterations in brain response via fMRI scanning. More specifically, these studies have demonstrated an increase in spatial working memory (SWM) brain response in marijuana users compared to normal age-matched controls in the prefrontal cortex, anterior cingulate, and the basal ganglia (Kanayama, Rogowska, Pope, Gruber, & Yurgelun-Todd, 2004). This suggests a compensatory neural response as well as recruitment of additional brain areas to achieve necessary task requirements, as seen in a recent study of task performance and brain functioning in marijuana users (Quickfall & Crockford, 2006). However, because the Kanayama et al. study was done on adults who were abstinent for only 6–36 hr prior to the scan, it may be that these effects reflect recent use and not persisting effects (Pope et al., 2001). Others have characterized visuospatial attention among 12 recent marijuana users who had used 2–24 hr earlier, 12 abstinent users who had not used for an average of 38 months, and 19 nonusing controls (Chang, Yakupov, Cloak & Ernst 2006). Both active and abstinent users showed decreased brain response in prefrontal, parietal, and cerebellar regions that normally subserve visual attention and increased activation in alternate regions, suggesting brain response alterations even after extended abstinence. These adult fMRI studies point to altered neural functioning among marijuana using adults during visuospatial tasks, particularly in frontal and parietal regions.
Less is known about neurocognitive functioning in adolescent marijuana users. A longitudinal study of 10 adolescent marijuana users showed incomplete recovery of learning and memory impairments even after 6 weeks of abstinence (Schwartz, Gruenewald, Klitzner, & Fedio, 1989). Recent fMRI studies of SWM involving alcohol-abusing adolescents and marijuana- and alcohol-abusing adolescents have found that marijuana and alcohol were associated with greater changes than alcohol alone. Specifically, after an average of 8 days of abstinence, adolescent marijuana users showed an increase in dorsolateral prefrontal activation and reduced inferior frontal response compared to alcohol users and nonusing controls, suggesting compensatory working memory and attention activity associated with heavy marijuana use during youth (Schweinsburg, Schweinsburg, et al., 2005). Adolescent marijuana users demonstrated increased right hippocampal activity and poorer attention and verbal working memory performance compared to demographically similar tobacco smokers and non-using controls, suggesting compensatory neural recruitment, even after a month of abstinence (Jacobsen, Mencl, Westerveld, & Pugh, 2004). In a follow-up study, marijuana-using youths who were abstinent at least 2 weeks performed similarly to nonusers on verbal working memory during ad libitum smoking and again during nicotine withdrawal; but they exhibited increased parietal activation and poorer verbal delayed recall during nicotine withdrawal compared to non-marijuana users (Jacobsen, Pugh, Constable, Westerveld, & Mencl, 2006). Together, these studies suggest altered working memory functioning among adolescent marijuana users that may persist after a month of abstinence. Yet it is unclear how variability in task performance might contribute to brain activation patterns.
Among normal adolescents, SWM task performance is associated with activation in bilateral prefrontal and posterior parietal brain regions (Klingberg, Forssberg, & Westerberg, 2002); Schweinsburg, Nagel, & Tapert, 2005; Thomas et al., 1999. Adult studies have suggested increased frontal and parietal activity associated with greater SWM task difficulty (Diwadkar, Carpenter, & Just, 2000; Jansma, Ramsey, Coppola, & Kahn, 2000; Leung, Seelig, & Gore, 2004). Results of fMRI studies of adolescent and adult marijuana users have suggested that increased neural responding associated with marijuana use may be evidence of compensatory neural recruitment to maintain task performance (Chang et al., 2006; Jacobsen et al., 2004, 2006; Kanayama et al., 2004; Quickfall & Crockford, 2006; Schweinsburg, Schweinsburg, et al., 2005). Therefore, the relationship between task performance and neural response may differ between marijuana users and controls, with a stronger positive relationship among marijuana users. The interaction between task performance and fMRI response to SWM has not yet been studied in adolescent marijuana users.
The goal of the present study was to understand how task performance patterns contribute to neural activation in abstinent adolescent marijuana users. We studied blood oxygen level-dependent (BOLD) fMRI neural activation during a SWM task that typically activates bilateral prefrontal and posterior parietal networks in adolescents and adults (Schweinsburg, Nagel, & Tapert, 2005; Tapert et al., 2001, 2004; Thomas et al., 1999; Wager & Smith, 2003). This SWM task has been shown to be sensitive to brain response abnormalities in adolescent alcohol (Tapert et al., 2004) and marijuana (Schweinsburg, Schweinsburg, et al., 2005) users. In this study, both adolescent users and controls were required to abstain from all drugs and alcohol for 28 days prior to their fMRI scan, and all were free from psychiatric disorders and learning disabilities.
Based on our previous work (Schweinsburg, Schweinsburg, et al., 2005), we predicted that after 28 days of abstinence, marijuana users as a group would perform as well as controls; however, the task performance would vary within each group, resulting in a Group × Task Performance interaction that would be associated with brain response. Specifically, we hypothesized that there would be interactions between task performance and fMRI response in the bilateral dorsolateral prefrontal and posterior parietal cortices, such that marijuana users would show a stronger positive association between performance and brain response than controls in these regions.
Method
Participants
Flyers were distributed at local high schools, community colleges, and universities to recruit 16–18-year-old adolescents (Tapert et al., 2003). Adolescent participants provided written informed assent (if age 16 or 17 years) or consent (if age 18 years) for their participation, and guardians (usually a parent) provided consent for youths under age 18 years. The University of California San Diego Human Research Protections Program approved this study. Participants were initially screened for eligibility and then were given a 45-min phone interview to collect information about general health, psychiatric disorders, and lifetime substance use. Participant parents gave consent for their own participation and were interviewed for detailed information about family health history, prenatal conditions, and their adolescent’s health and developmental history. The computerized National Institute of Mental Health Diagnostic Interview Schedule for Children Predictive Scale (DISC-PS-4.32b; Lucas et al., 2001; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000) was conducted separately with the youth and a parent to exclude adolescents with a potential psychiatric disorder (including conduct disorder, attention-deficit/hyperactivity disorder, and substance use disorders). Other exclusion criteria included prenatal substance exposure, birth complications, psychotropic medication use, physical health problems, neurological dysfunction, head injury, family history of bipolar I or psychotic disorder (collected with the Family History Assessment Module screener; Rice et al., 1995), left-handedness, learning disability, or MRI incompatibility. Teens found to meet diagnostic criteria for alcohol use disorder were not excluded due to high comorbidity with marijuana use (Agosti, Nunes, & Levin, 2002). Two subjects, both in the marijuana group, met criteria for alcohol abuse.
Groups consisted of 17 heavy marijuana users and 17 nonusing demographically similar controls. Users reported 477 episodes of lifetime marijuana use, on average, and control participants reported no more than 5 lifetime uses of marijuana. Groups were comparable in age, gender, ethnicity, family history of substance use disorders, and depressed and anxious mood (see Table 1). Marijuana users and control teens showed similar levels of IQ, as prorated by the Wechsler Abbreviated Scale of Intelligence Vocabulary and Block Design subtests (Ryan et al., 2005; Wechsler, 1999) and socioeconomic status (Hollingshead, 1965). Even though marijuana users reported more use of other drugs than controls, lifetime use of other drugs was fewer than 27 times across all substance types besides nicotine, alcohol, and marijuana. Marijuana users reported higher rates of alcohol than controls, and both groups had low rates of tobacco use.
Table 1.
Characteristics of Adolescent Participants
| Characteristic | MJ (n = 17) | Controls (n = 17) |
|---|---|---|
| Age in years (range = 16–18) | 18.06 (0.75) | 17.9 (1.12) |
| Female (%) | 18 | 29 |
| Family history negative (%)a | 53 | 77 |
| CBCL Externalizing t score | 47.31 (5.73) | 44.48 (7.05) |
| CBCL Internalizing t score | 46.74 (7.57) | 46.89 (8.83) |
| Beck Depression Inventory | 4.41 (7.07) | 1.76 (2.59) |
| Spielberger State Anxiety t score | 38.04 (8.39) | 40.74 (10.20) |
| Parent annual salary (thousands of dollars) | 116.35 (84.03) | 121.00 (73.50) |
| WASI Vocabulary t score | 55.53 (8.74) | 55.53 (7.93) |
| WASI Block Design t score | 59.82 (5.67) | 54.35 (11.19) |
| Lifetime alcohol use episodes | 147.35 (125.26)* | 9.94 (30.71) |
| Average cigarettes per day | 1.59 (1.67) | 0.35 (7.21) |
| Lifetime marijuana use episodes | 477.06 (260.07)* | 0.53 (1.33) |
Note. Except as indicated, data are means (SD). MJ = marijuana using teens with 28 days of abstinence; CBCL = Child Behavior Checklist; WASI = Wechsler Abbreviated Scale of Intelligence.
No first-degree biological relative with alcohol or drug abuse or dependence.
p <.0001.
Measures
Substance use
Substance intake was assessed using the Customary Drinking and Drug Use Record (Brown et al., 1998). Self-reported information was collected about lifetime and past-3-month use of marijuana, alcohol, nicotine, and other drugs. Strong internal consistency, test–retest, and interrater reliability have been shown with adolescent Customary Drinking and Drug Use Record assessments (Brown et al., 1998; Stewart & Brown, 1995). The timeline follow-back technique was used to assess drug and alcohol use for the previous 28 days. Participants were asked to point out for each day whether they used or drank. If they disclosed use, they were to indicate how many hits of marijuana or drinks of alcohol they consumed (Sobell & Sobell, 1992). “Drinks” were defined as “one can of beer, one glass of wine, or one shot of hard liquor” to clarify the amount of alcohol consumed. If asked, a “hit” of marijuana was defined as “a puff from a pipe, bong, joint, or vaporizer.” because smoking is the most common method of use.
State scales
The Beck Depression Inventory (Beck, 1978) and the Spielberger State Trait Anxiety Inventory (Spielberger, Gorsuch, & Lushene, 1970) measured mood prior to the time of scanning. The Stanford Sleepiness Scale (Glenville & Broughton, 1978) determined alertness immediately before and after scanning with self-report ratings (1 = alert to 7 = almost asleep).
Psychopathological syndromes
Parents were interviewed about the child’s internalizing and externalizing behaviors via the Child Behavior Checklist (Achenbach & Rescorla, 2001).
SWM task
This task (Kindermann, Brown, Zorrilla, Olsen, & Jeste, 2004; Tapert et al., 2001) consisted of eighteen 21-s blocks that alternated between resting fixation, vigilance, and SWM conditions (see Figure 1). Each block began by showing a 1-s word cue that prompted the upcoming block. The resting fixation block began with the cue “LOOK,” and subjects were asked to look at the fixation cross. Each vigilance block was prompted by the cue “DOTS,” and subjects were asked to respond with a button press to figures that had a dot above them (30% of the figures). Before each working memory block, the cue “WHERE” appeared on the screen. During these blocks, abstract figures were individually shown in one of eight spatial locations, and subjects were instructed to respond with a button press every time a figure appeared in the same location as a previous figure had been within that block. Unknown to the subject, repeat location stimuli were 2-back, and composed 30% of stimuli. For both the vigilance and SWM conditions, stimuli were presented for 1000 ms, with an interstimulus interval of 1000 ms (21 s per block; total task time = 7 min, 48 s; see Figure 1). All subjects were given practice with the task prior to entering the scanner and were monitored to ensure they understood the task instructions. Performance data were collected for accuracy and reaction time with a fiber optic response box.
Figure 1.
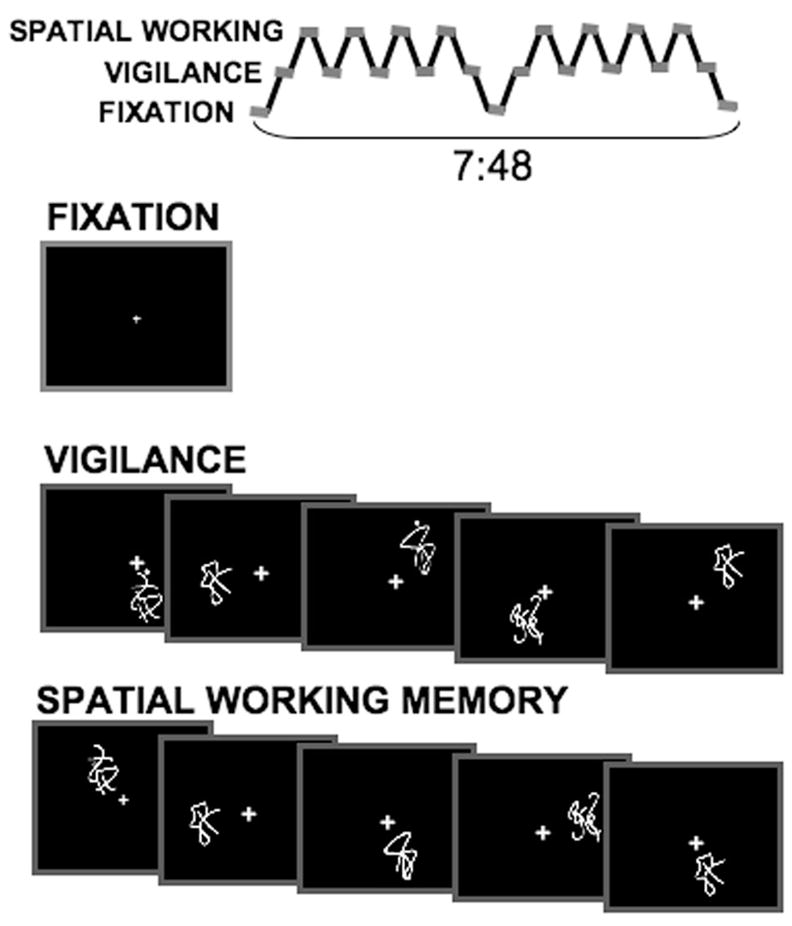
The spatial working memory task design.
Procedures
Toxicology
The toxicology procedure was designed to ensure that participants would not use substances in the 28 days prior to the fMRI scan. Cannabis metabolites can reliably remain detectible in urine for at least 4 days (Fraser, Coffin, & Worth, 2002). Subjects were required to give a urine sample every 3–4 days each week during the 28 days prior to the fMRI session to make sure there was no recent use of cannabis, amphetamines, methamphetamines, benzodiazepines, cocaine, barbiturates, codeine, morphine, phencyclidine, or ethanol. Samples were collected and analyzed at the Veterans Affairs Medical Center using cloned enzyme donor immunoassay kits. Collections were observed to minimize the risk of participant tampering. Quantitative indices were tracked to determine if tetrahydrocannabinol metabolite levels decreased during the 28-day period. Participants who initially screened positive for cannabis were accepted and retained, as long as tetrahydrocannabinol metabolite indices decreased continually throughout the 28 days. If participant’s levels increased or if a positive screen was obtained after a negative screen, the participant was given the option to restart the 28-day abstinence period or drop out of the study. All participants produced negative urine toxicology screens at the time of scanning. Breathalyzers checked for recent alcohol use prior to the fMRI scan.
Imaging
Anatomical and functional imaging data were acquired with a 1.5 Tesla Signa LX scanner (General Electric Corp., Milwaukee, WI). The high-resolution structural scan was collected in the sagittal plane using an inversion recovery prepared T1-weighted 3D spiral fast spin echo sequence (TR = 2,000 ms, TE = 16 ms, FOV = 240 mm, 256 × 256, resolution = 0.94 × 0.94 mm × 1.33 mm, 128 continuous slices, acquisition time = 8:36; Wong, Luh, Buxton, & Frank, 2000). The functional scan was acquired in the axial plane using T2*-weighted spiral gradient recall echo imaging (TR = 3,000 ms, TE = 40 ms, flip angle = 90°, FOV = 240 mm, 64 × 64, 19–21 slices covering the whole brain, slice thickness = 7 mm, reconstructed in-plane resolution = 1.88 × 1.88 mm, 156 repetitions).
Data Analyses
Task performance
SWM task reaction time and accuracy data were collected during scanning, and composite scores were calculated to provide a single, comprehensive measure of performance and use fewer degrees of freedom in analyses, providing more statistical power (Rympa, Berger, & D’Esposito, 2002). Reaction-time data and accuracy measures were converted into z scores, and reaction-time z scores were subtracted from accuracy z scores to compute the performance composite score. Using this approach, high accuracy would result in a high positive z score, whereas low reaction time, which is better, would result in a high negative z score. Therefore subtraction of the negative reaction time z score from the positive accuracy z score would yield a positive index indicating high overall performance.
Imaging data
Imaging data from each participant were processed as in our prior studies (e.g., Tapert et al., 2003, 2004; Schweinsburg, Nagel, & Tapert, 2005; Schweinsburg, Schweinsburg, et al., 2005), using Analysis of Functional NeuroImages (Cox, 1996). Time series data were corrected for motion. Number of removed repetitions and average movement in each direction throughout the task were examined in relation to group, task, and interactions using correlational analyses. The average percent of repetitions removed for excessive motion during the task was 8%, resulting in 92% retained for analyses. There were no significant differences between groups in bulk motion in any of the six movement directions (roll, pitch, and yaw rotations; superior, left, and posterior displacements). The average rotational movement throughout the task for marijuana users was 0.04, 0.14, and 0.05 degrees for roll, pitch, and yaw, respectively. In controls, the average rotational movement throughout the task was 0.07, 0.13, and 0.06 degrees for roll, pitch, and yaw, respectively. Among marijuana users, the average translational movement was 0.11, 0.05, and 0.08 mm for superior, left, and posterior, respectively; the average translational movement of controls was 0.14, 0.06, and 0.07 mm for superior, left, and posterior, respectively. There was a significant group difference in the roll direction, t(32) = 2.35, p = .03, although such movements were quite small.
Next, fMRI data were deconvolved with a reference function that coded the hypothesized BOLD signal for each task condition (see Figure 1; Cox & Jesmanowicz, 1999; Ward, 2002). Controlling for linear trends, spin history effects, and delays in hemodynamic response, we computed for each brain voxel a fit coefficient that represented the relationship between the observed and hypothesized signal change for contrasts between SWM and vigilance conditions (Friston, Williams, Howard, Frackowiak, & Turner, 1996). These functional datasets were warped into standard space (Talairach & Tournoux, 1988), resampled into 3 mm3 voxels, and smoothed with a 5 mm Gaussian filter.
Statistical analyses
Regression analyses determined the variability in brain response accounted for by group and task performance and their interaction. These group-level analyses were performed in each voxel of the brain and examined the BOLD response contrast between SWM and vigilance. To control for Type 1 error, we only interpreted significant effects in clusters of 50 contiguous significant voxels ( p <.05; 1358μl in volume), yielding an overall clusterwise alpha of .05, determined by Monte Carlo simulations (Ward, 2000). Exploratory follow-up regression analyses were performed to determine the nature of the Group × Performance Interaction.
Results
Behavioral Performance
SWM task performance data were available for all 34 subjects. The vigilance condition revealed an average accuracy of 97 ± 1.84% (mean ± SD) for users and 96 ± 2.39% for controls. Reaction times for vigilance were 623 ± 42 ms for users and 637 ± 66 ms for controls. The SWM condition demonstrated an average accuracy percentage of 94 ± 5.26 in users and 93 ± 6.11 for controls, and reaction times were 540 ± 84.93 ms in users and 548 ± 85.48 ms in controls. Independent samples t tests to compare means showed no differences between the groups for either accuracy or reaction times in the vigilance and SWM conditions. The vigilance composite score was 0.51 ± 1.03 for users and −0.02 ± 1.74 for controls. For SWM, the marijuana users scored 0.01 ± 1.29 and controls scored 0.27 ± 1.29. Within-subjects analyses revealed that there was no significant difference in composite scores between vigilance and SWM conditions, F(1, 32) = 0.09, p < .76, and no Group × Task Condition (SWM vs. vigilance) interaction, F(1, 32) = 1.20, p <.28. Between-subjects analyses demonstrated no group differences, F(1, 32) = 0.21, p < .65.
FMRI Response
A main effect of group revealed that marijuana users showed significantly ( p < .05) greater activation than controls in a cluster encompassing the right basal ganglia, as well as in a second cluster encompassing the right precuneus, postcentral gyrus, and superior parietal lobule (Brodmann’s Area 7) and in the left precuneus and superior parietal lobule (Brodmann’s Area 7). There was no region in which marijuana users demonstrated reduced activation compared to controls (Table 2).
Table 2.
Blood Oxygen Level-Dependent Response Differences to the Spatial Working Memory Task Between Abstinent Marijuana Users and Control Adolescents
| Talairach coordinatesa |
||||||
|---|---|---|---|---|---|---|
| Relationship and anatomic region | Brodmann’s areas | Volume (μl) | x | y | z | t statisticb |
| Main effect for group | ||||||
| MJ > Controls | ||||||
| Right claustrum, putamen, caudate, thalamus, globus pallidus, insula, globus pallidus | 3,024 | 32 R | −17 P | 12 S | 2.27 | |
| Right precuneus, superior parietal lobule, postcentral gyrus | 7 | 2,943 | 8 R | −53 P | 69 S | 4.47 |
| Left superior parietal lobule, precuneus | 7 | 2,133 | −11 L | −65 P | 63 S | 4.29 |
|
| ||||||
| Main effect for performance | ||||||
| Positive relationship | ||||||
| Right middle and inferior temporal gyrus, parahippocampal gyrus | 20, 21, 36 | 8,802 | 62 R | −41 P | −4 I | 3.17 |
| Right cerebellar tonsil | 5,184 | 8 R | −32 P | −46 I | 7.07 | |
| Right inferior parietal lobule, supramarginal gyrus, angular gyrus, middle temporal gyrus | 39, 40 | 4,131 | 41 R | −53 P | 51 S | 2.56 |
| Left middle and superior temporal gyrus | 21, 22 | 3,267 | −56 L | −41 P | −1 I | 2.99 |
| Left middle occipital gyrus, middle and inferior temporal gyrus | 37, 39 | 1,512 | −50 L | −62 P | −7 I | 2.48 |
| Right middle frontal gyrus | 9 | 1,458 | 47 R | 14 A | 33 S | 3.40 |
| Left middle and inferior frontal gyrus | 47 | 1,404 | −50 L | 35 A | −4 I | 3.27 |
|
| ||||||
| Interaction of Group × Performance | ||||||
| Left superior temporal lobule, superior and middle temporal gyrus | 13, 41 | 2,700 | −59 L | −41 P | 6 S | 2.71 |
| Right superior temporal gyrus, uncus | 38 | 2,187 | 32 R | 2 A | −34 I | 3.70 |
| Left anterior cingulate | 32 | 1,917 | −2 L | 26 A | 4 S | 5.30 |
| Left uncus, parahippocampal gyrus | 28, 35 | 1,593 | −23 L | −8 P | −28 I | 3.62 |
| Right thalamus | 1,539 | 23 R | −29 P | 6 S | 1.75 | |
Note. MJ = marijuana using teens with 28 days of abstinence; R = right; L = left; A = anterior; P = posterior; S = superior; I = inferior.
Talairach coordinates refer to maximum signal intensity group difference or relationship within the cluster.
t statistic represents maximum intensity t value of all voxels within the cluster.
Across all subjects, both users and controls, behavioral performance data positively predicted activation in seven clusters (see Table 2): (a) right middle temporal gyrus, parahippocampal gyrus, and inferior temporal gyrus; (b) right cerebellar tonsil; (c) right inferior parietal lobule, supramarginal gyrus, angular gyrus, and middle temporal gyrus; (d) left middle temporal gyrus and superior temporal gyrus; (e) left middle occipital gyrus, middle temporal gyrus, and inferior temporal gyrus; (f) right middle frontal gyrus; and (g) left middle frontal gyrus and inferior frontal gyrus. There were no regions in which performance was negatively associated with brain response (see Table 2).
A Group × Performance Interaction was found in five clusters: (a) left superior temporal lobule, left superior temporal gyrus, and left middle temporal gyrus; (b) right temporal gyrus and right uncus; (c) left anterior cingulate; (d) left uncus and left parahippocampal gyrus; and (e) right thalamus and right pulvinar. We observed positive associations between SWM response and task performance in users and negative associations for controls in the first, F(3, 30) = 7.92, p < .0001, r2 = 36%, (see Figure 2) and third, F(3, 30) = 6.33, p < .002; R2 = 31%, (see Figure 3) clusters. A negative association among users and a positive association in controls were revealed in the second, F(3, 30) = 4.97, p < .006, R2 = 27%, (see Figure 4); fourth, F(3, 30) = 5.5, p < .004, R2 = 35%, (see Figure 5); and fifth, F(3, 30) = 4.39, p < .011, R2 = 29%, (see Figure 6) clusters.
Figure 2.
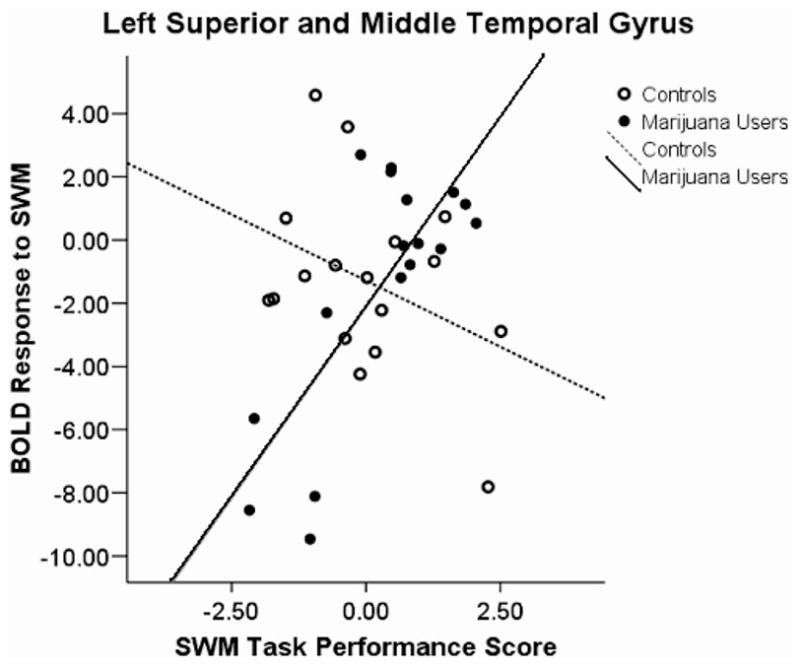
Blood oxygen level-dependent (BOLD) response interactions to the spatial working memory (SWM) task in the left superior and middle temporal gyrus.
Figure 3.
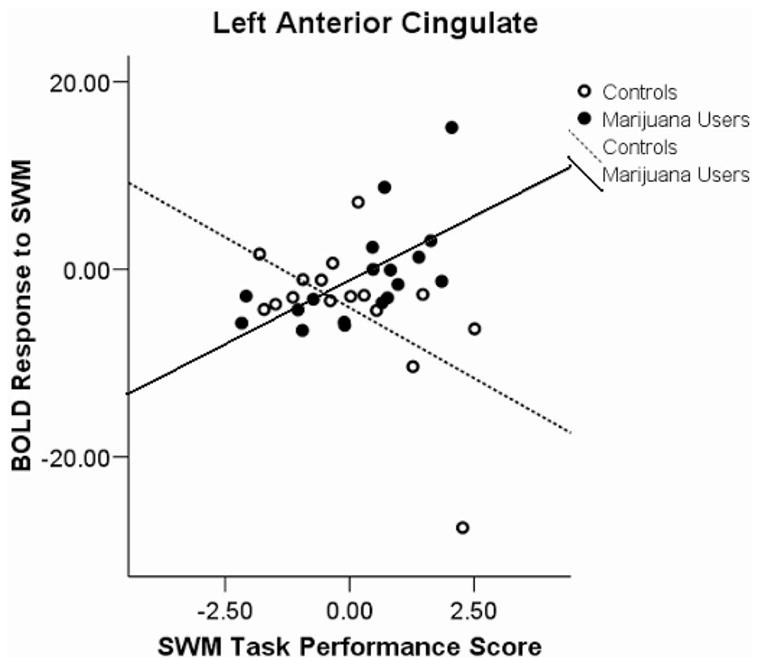
Blood oxygen level-dependent (BOLD) response interactions to the spatial working memory (SWM) task in the left anterior cingulate.
Figure 4.
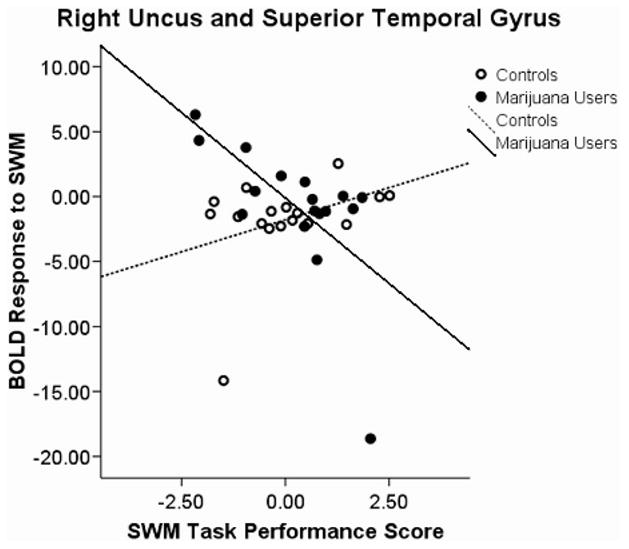
Blood oxygen level-dependent (BOLD) response interactions to the spatial working memory (SWM) task in the right uncus and superior temporal gyrus.
Figure 5.
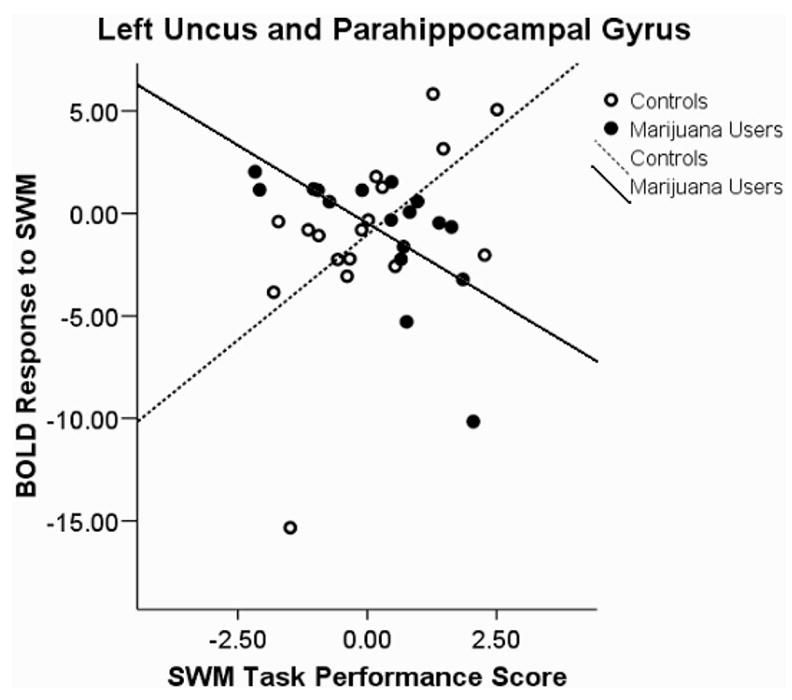
Blood oxygen level-dependent (BOLD) response interactions to the spatial working memory (SWM) task in the left uncus and parahip-pocampal gyrus.
Figure 6.
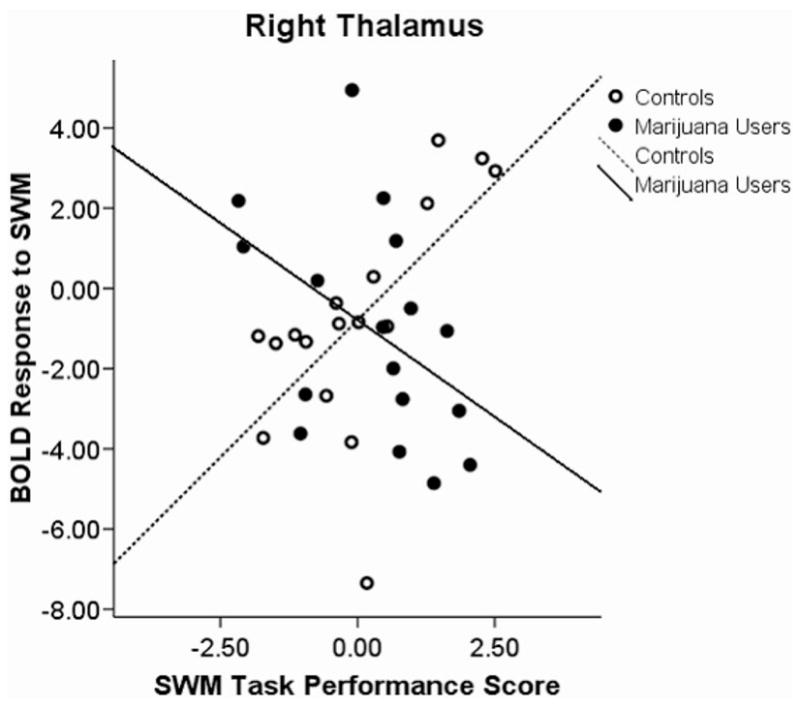
Blood oxygen level-dependent (BOLD) response interactions to the spatial working memory (SWM) task in the right thalamus.
There was a group difference in movement found in the right basal ganglia in the roll direction, t(32) = 2.35, p = .03. However, findings were reexamined using movement as a covariate, and all findings remained unchanged ( ps < .025). Performance and BOLD response data were checked for outliers, and none were found. Cases appearing as possible outliers on scatterplots were removed and analyses were redone; results remained unchanged. Both groups were checked for outliers on mood measures; although neither group contained an outlier on the Beck Depression Inventory, the marijuana group contained one outlier on the Hamilton Anxiety Rating Scale. Analyses were rerun excluding this subject and results remained unchanged.
Discussion
This study examined the association between behavioral performance and brain response during a SWM task among 16–18-year-old marijuana users and controls after 28 days of abstinence. Results suggest that, in general, marijuana-using teens performed similarly to controls on SWM, perhaps due to the low difficulty level of the task (only eight spatial locations and 2-back working memory load), which approached ceiling effects. This has been observed in fMRI studies of SWM in adult marijuana users (Kanayama et al., 2004). However, specific localization and intensity of response varied between the marijuana users and controls, with marijuana users showing more performance-related activation in certain regions and less in others. These differential patterns emerged despite similar overall task performance across groups, suggesting an alternate relationship between task performance and brain activity among marijuana users.
Marijuana users showed significantly more activation than controls in the right basal ganglia, an area associated with skill-learning (Halsband & Lange, 2006). Because the subjects were only allowed to practice the task once before entering the scanner, it is possible that the marijuana users were still in the skill learning process during imaging. The other two clusters, which were significantly more activated in marijuana users than controls, were the right and left parietal lobes. Bilateral parietal regions have been implicated in attention, spatial perception, imagery, working memory, spatial encoding, episodic retrieval, skill learning monitoring, organization, and planning during working memory (Cabeza & Nyberg, 2000; Wager & Smith, 2003). It is possible that there is compensatory neural effort in these areas, as observed in SWM studies of adult marijuana users (Kanayama et al., 2004).
The performance data positively related to activation in several areas and did not negatively associate with brain response in any region. Performance was positively associated with activation in the left and right temporal regions, which are associated with verbal mechanisms and episodic, nonverbal working memory and retrieval, respectively (Cabeza & Nyberg, 2000). This suggests that good task performance may be related to using multiple memory modalities. High scorers showed more activation in the bilateral prefrontal and bilateral parietal regions that have been shown to activate during SWM tasks in youths (Thomas et al., 1999; Schweinsburg, Nagel, & Tapert, 2005).
The Performance × Group interactions were the focus of this study and yielded the most interesting results. In particular, an interaction in the left superior temporal gyrus suggested a positive association in the users and a negative association in the controls. This may imply that the marijuana users employed more of a verbal strategy to achieve high task performance scores than the controls. This is interesting when considering the previous findings of deficits in verbal learning and IQ in marijuana using adolescents compared to controls (Fried, Watkinson, & Gray, 2005).
Furthermore, the right superior temporal gyrus showed an interaction where users had a negative association and controls had a positive association. Previous studies have shown this area to be involved in poorer recognition of previously seen words (de Zubicaray, McMahon, Eastburn, Finnigan, & Humphreys, 2005). This would support the notion that users are relying on a verbal strategy such that better performance linked to a decrease in activation in the right superior temporal gyrus. Moreover, an interaction in the right thalamus and pulvinar showed a negative association in the users and a positive association in the controls. These subcortical structures have shown an association with spatial neglect when damaged (Karnath, Himmelbach, & Rorden, 2002). It is interesting that these areas have a negative association in users and a positive association in controls and may suggest that marijuana users utilize less spatial strategies than controls.
The nature of the interaction revealed a positive association in marijuana users and a negative association in controls in the left anterior cingulate. This region has been linked to attention, decision making, cue response, and response monitoring (Ansari, Fugelsang, Dhital, & Venkatraman, 2006; Dosenbach et al., 2006). It may be that good performing marijuana users are making a more conscious decision to react to task cues than controls, who may be reacting more automatically. The left parahippocampal gyrus demonstrated an interaction of negative association in marijuana users and positive association for controls. This region is involved in working memory and is recruited when the temporal lobe is not in use (Yetkin, Rosenberg, Weiner, Purdy, & Cullum, 2006). Because marijuana users are using more energy in the left temporal lobe as their performance increases, higher scoring subjects may rely less on the parahippocampal gyrus.
The distinct interactions viewed in these different areas of the brain can mean that different systems are at work and that, as one part of a system decreases in action, the other area of the system increases in activation. Previous studies suggest that subjects who do not use traditional strategies for specific tasks showed an increased extent of activation and recruitment of additional areas, specifically verbal areas, to accomplish the task (Kindermann et al., 2004; Yetkin et al., 2006). More specifically, the pattern of results suggests that marijuana users may apply a verbal strategy to the task when achieving higher scores. It is possible that this alternative way of using the brain may be less efficient; this would explain the greater overall activation in users versus controls and recruitment of other brain regions as a compensation method. A recent review also found that multiple neuroimaging studies of marijuana users pointed toward recruitment of compensatory regions as well as task-related regions to achieve task demands (Quickfall & Crockford, 2006).
A possible limitation to this study is the interpretation of a difference in fMRI activation between experimental groups. It is possible that alternative neural pathway use is more dynamic and versatile. It is unclear whether the results are an adverse effect of the marijuana use or merely a benign difference. Further studies that more carefully describe the relationships between task performance and brain response will clarify this question. Another limitation of the current study is that most marijuana users were also moderately heavy alcohol drinkers. Although these participants are representative of the population of adolescent marijuana users, most of whom also consume alcohol (Agosti et al., 2002), it is nonetheless difficult to disentangle the effects that may be related to alcohol use. Alcohol use correlated with brain response in the right thalamus and pulvinar in the current study, but results remained significant even when accounting for alcohol use, and alcohol use did not correlate to activation in any other significant regions. Our previous research identified brain response abnormalities among marijuana users above and beyond those demonstrated by users of alcohol alone (Schweinsburg, Schweinsburg, et al., 2005), supporting the hypothesis of marijuana-specific differences in brain response, even among teens who are heavy drinkers. Future studies should attempt to clarify the differential and interactive impact of concomitant alcohol and marijuana use on brain functioning on adolescents. Furthermore, lifetime marijuana use episodes were associated with activation in the right uncus and superior temporal gyrus. Future analyses could further investigate the associations of other brain regions, as well as neuropsychological performance, with lifetime use episodes. These subtle differences among users may provide additional insight into the mechanisms involved with prolonged abstinence from marijuana.
Future studies should also focus on investigating the nature of interactions in other domains of cognition to test if other types of tasks show these patterns. A more complex task should be an aim for future studies because it may elicit a difference in task performance. If a user’s neural differences are actually a compensatory tool, then a more difficult task may overcome their compensation abilities, therefore resulting in performance deficits. In addition, a parametric manipulation of working memory load could help specify degree of compensatory activation in marijuana users compared to controls, as marijuana users may reach a limit earlier than controls. Further studies could also explore which mechanisms and strategies subjects utilize during the tasks through qualitative data investigation.
Acknowledgments
This experiment was conducted as part of an honors thesis by Claudia B. Padula at the University of California, San Diego. This research was supported by National Institute on Drug Abuse Grants DA15228 and DA021182 to Susan F. Tapert. We wish to thank John Polich, Victor Ferreira, Ebbe Ebbesen, M. J. Meloy, Ann Park, Tim McQueeny, Bonnie Nagel, Omid Khalili, Gloria Padula, and Daniel Padula for guidance, assistance, and support in every aspect of this project.
References
- Achenbach TM, Rescorla L. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont; 2001. [Google Scholar]
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. American Journal of Drug and Alcohol Abuse. 2002;28:643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Ansari D, Fugelsang JA, Dhital B, Venkatraman V. Dissociating response conflict from numerical magnitude processing in the brain: An event-related fMRI study. NeuroImage. 2006;32:799–805. doi: 10.1016/j.neuroimage.2006.04.184. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) San Antonio, TX: Psychological Corporation; 1978. [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Chen K, Sheth AJ, Elliot DK, Eagerm A. Prevalence and correlates of past-year substance use, abuse, and dependence in a suburban community sample of high-school students. Addictive Behaviors. 2004;29:413–423. doi: 10.1016/j.addbeh.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. Retrieved from http://afni.nimh.nih.gov/afni/ [DOI] [PubMed]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Mackay AJ, Mills AT, Gruzelier JG. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology. 2001;153:373–379. doi: 10.1007/s002130000591. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, McMahon KL, Eastburn MM, Finnigan S, Humphreys MS. fMRI evidence of word frequency and strength effects in recognition memory. Cognitive Brain Research. 2005;24:587–598. doi: 10.1016/j.cogbrainres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Carpenter PA, Just MA. Collaborative activity between parietal and dorso-lateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. NeuroImage. 2000;12:85–99. doi: 10.1006/nimg.2000.0586. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from disuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: What have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Fraser AD, Coffin L, Worth D. Drug and chemical metabolites in clinical toxicology investigations: The importance of ethylene glycol, methanol and cannabinoid metabolite analyses. Clinical Biochemistry. 2002;35:501–511. doi: 10.1016/s0009-9120(02)00325-9. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana—A comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861– 863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glenville M, Broughton R. Reliability of the Stanford Sleepiness Scale compared to short duration performance tests and the Wilkinson Auditory Vigilance Task. Advances in the Biosciences. 1978;21:235–244. [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: A review of functional and clinical studies. Journal of Physiology, Paris. 2006;99:414 – 424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Computational Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis on brain function in adolescents. Annals of the New York Academy of Sciences. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry. 2006;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Coppola R, Kahn RS. Specific versus nonspecific brain activity in a parametric N-back task. NeuroImage. 2000;12:688–697. doi: 10.1006/nimg.2000.0645. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age–consistency and interpretation of observed effects. Neurobiology of Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. The Monitoring the Future national results on adolescent drug use: Overview of key findings, 2005. Bethesda, MD: National Institute on Drug Abuse; 2006. [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: A functional magnetic resonance imaging study. Psychopharmacology. 2004;16:16. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: Putamen, caudate nucleus and pulvinar. Brain: A Journal of Neurology. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Kindermann SS, Brown GG, Zorrilla LE, Olsen RK, Jeste DV. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophrenia Research. 2004;68:203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neurosciences. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Leung HC, Seelig D, Gore JC. The effect of memory load on cortical activity in the spatial working memory circuit. Cognitive, Affective & Behavioral Neurosciences. 2004;4:553–563. doi: 10.3758/cabn.4.4.553. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Jacobs A, Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuo-spatial memory. Psychotherapy and Psychosomatics. 1997;66:179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. Journal of the American Medical Association. 1996;275:521–527. [PubMed] [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: A review. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:318–332. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Carruthers CA, Miller LJ, Souheaver GT, Gontkovsky ST, Zehr MD. The WASI matrix reasoning subtest: Performance in traumatic brain injury, stroke, and dementia. International Journal of Neurosciences. 2005;115:129–136. doi: 10.1080/00207450490512704. [DOI] [PubMed] [Google Scholar]
- Rympa B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. Journal of Cognitive Neuroscience. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. American Journal of Diseases in Children. 1989;143:1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescent development. Journal of the International Neuropsychological Society. 2005;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas C, Dulcan M, Schwab-Stone M. NIMH Diagnostic Interview Schedule for Children version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2005 National Survey on Drug Use and Health: National Findings (NHSDA Series H-30, DHHS Publication No. SMA 06–4194) Rockville, MD: Office of Applied Studies; 2006. [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Three-dimensional proportional system: An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism, Clinical and Experimental Research. 2004;28:1577–1578. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, et al. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective & Behavioral Neurosciences. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Milwaukee: Biophysics Research Institute, Medical College of Wisconsin; 2000. [Google Scholar]
- Ward BD. Deconvolution analysis of fMRI time series data. Milwaukee: Biophysics Research Institute, Medical College of Wisconsin; 2002. [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank RL. Single slab high resolution 3D whole brain imaging using spiral FSE. Proceedings of the International Society on Magnetic Resonance in Medicine. 2000;8:683. [Google Scholar]
- Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. European Radiology. 2006;16:193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]


