Abstract
Background
Meningococcal outer membrane vesicle (OMV) vaccines are efficacious in humans but have serosubtype-specific serum bactericidal antibody responses directed at the porin protein PorA and the potential for immune selection of PorA-escape mutants.
Methods
We prepared an OMV vaccine from a Neisseria meningitidis strain engineered to overexpress genome-derived neisserial antigen (GNA) 1870, a lipoprotein discovered by genome mining that is being investigated for use in a vaccine.
Results
Mice immunized with the modified GNA1870-OMV vaccine developed broader serum bactericidal antibody responses than control mice immunized with a recombinant GNA1870 protein vaccine or an OMV vaccine prepared from wild-type N. meningitidis or a combination of vaccines prepared from wild-type N. meningitidis and recombinant protein. Antiserum from mice immunized with the modified GNA1870-OMV vaccine also elicited greater deposition of human C3 complement on the surface of live N. meningitidis bacteria and greater passive protective activity against meningococcal bacteremia in infant rats. A N. meningitidis mutant with decreased expression of PorA was more susceptible to bactericidal activity of anti-GNA1870 antibodies.
Conclusions
The modified GNA1870-OMV vaccine elicits broader protection against meningococcal disease than recombinant GNA1870 protein or conventional OMV vaccines and also has less risk of selection of PorA-escape mutants than a conventional OMV vaccine.
Outer membrane vesicle (OMV) vaccines elicit protective immunity against Neisseria meningitidis group B disease (reviewed in [1]). Recently, an OMV vaccine received a provisional license in New Zealand and was introduced for widespread immunization in response to a group B epidemic that has been ongoing there for more than a decade [2–4]. One important limitation of OMV vaccines is that they elicit bactericidal antibody responses that are largely directed against surface-exposed loops of PorA [5], a major porin protein, and there is considerable PorA antigenic diversity in strains causing endemic meningococcal disease [6]. Thus, OMV vaccines are of greatest use for prevention of epidemic disease caused by a predominant (clonal) meningococcal strain, such as in New Zealand [4].
Recent efforts to develop group B meningococcal vaccines have focused on antigenically conserved antigens, such as neisserial surface protein A (NspA) [7, 8], or a number of other novel proteins (referred to as “genome-derived neisserial antigens” [GNA]) discovered during the N. meningitidis MC58 genome sequencing project [9]. Among the latter is GNA1870, a lipoprotein of unknown function that is presently being evaluated for use in a recombinant protein vaccine [10, 11]. GNA1870 can be subdivided into 3 variant groups on the basis of amino-acid variability and antigenic cross-reactivity. Strains expressing GNA1870 in the variant 1 (v.1) group account for ~60% of the disease-producing group B isolates [11]. In a previous study, mice immunized with a recombinant GNA1870 (rGNA1870) v.1 protein vaccine developed serum bactericidal antibody responses against most, but not all, strains expressing subvariants of the GNA1870 v.1 protein [10]. Thus, GNA1870 is a promising antigen for inclusion in a protective meningococcal vaccine, but it would be desirable to improve the breadth of the protective antibody responses elicited by the recombinant protein.
In the present study, we investigated serum antibody responses elicited in mice after immunization with an OMV vaccine prepared from a N. meningitidis strain genetically engineered to overexpress GNA1870 v.1 protein. Our hypothesis was that the functional activity of antibodies elicited by the overexpressed native GNA1870 v.1 protein anchored in the OMV might be greater than that elicited by a rGNA1870 protein vaccine or by a conventional OMV vaccine.
MATERIALS AND METHODS
Bacterial strains
The 7 N. meningitidis strains used in this study are listed in table 1. Strain RM1090 naturally expresses low levels of a GNA1870 variant 2 (v.2) protein. The other 6 strains express subvariants of GNA1870 v.1 proteins [10, 11] and are genetically diverse on the basis of their genetic lineages as defined by electrophoretic cluster analysis [12, 13] and/or sequencing typing [14].
Table 1.
Summary of Neisseria meningitidis strains.
| Strain | Country of origin | Serologic classification | PorA VR sequence typea | Electrophoretic type (ET) cluster (sequence type [ST])b | GNA1870 variant group (% amino acid identity)c,d | Serum anti–rGNA1870protein bactericidal titere |
|---|---|---|---|---|---|---|
| Z1092 | Germany | A:4,21:P1.10 | 1.5–2,10 | Unknown (ST-1 complex/subgroup I/II) | 1 (96) | ND |
| BZ198 | The Netherlands | B:NST:P1.4 | 7–2,4 | ET154 (Unknown) | 1 (92) | ND |
| CU385 | Cuba | B:4,7:P1.19,15 | 19,15 | ET5 complex (ST-33) | 1 (100) | 1:2500 |
| M1390 | United States | B:15:P1.7,4 | ND | ET lineage 3 (ST-41) | 1 (92) | <1:10 |
| M6190 | United States | B:2a:P1.5,2 | ND | ET37 complex (ST-1988) | 1 (94) | <1:10 |
| NZ98/254 | New Zealand | B:4:P1.4 | 1.7–2,4 | ET lineage 3 (ST-42) | 1 (92) | <1:10 |
| RM1090 | United States | C:2a:P1.5,2 | 5–1,2 | Unknown | 2 (70) | ND |
NOTE. ND, not determined by either Moe et al. [16] or Welsch et al. [10]; rGNA1870, recombinant GNA1870; VR, variable region.
ST typing was performed by multilocus sequencing, as described elsewhere (available at: http://www.mlst.net).
Percentage of amino acid identity, compared with that of GNA1870 from strain MC58. Note that the respective amino acid sequences of GNA1870 in strains BZ198, M1390, and NZ98/254 are identical.
The 6 GNA1870 variant 1 protein strains showed similar respective GNA1870 protein expression as measured by Western blot, performed as described elsewhere [10].
Bactericidal titer of hyperimmune mouse antiserum raised against rGNA1870 given with Freund’s adjuvant and measured with human complement, as reported by Welsch et al. [10].
pFP12-GNA1870 shuttle vector construct
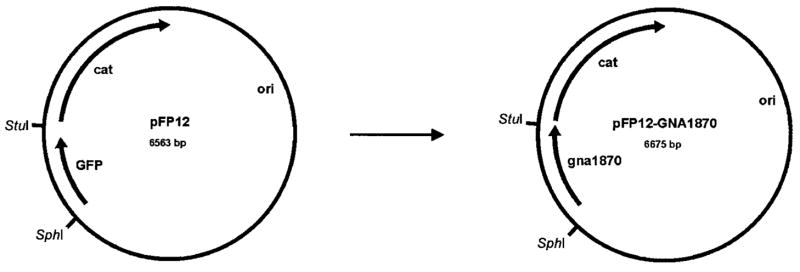
To overexpress GNA1870 v.1 protein in N. meningitidis, we used the shuttle vector pFP12, which has an origin of replication from a naturally occurring plasmid in N. gonorrhoeae [15] (gift from Jo-Anne Dillon, University of Saskatchewan, Saskatoon, Saskatchewan, Canada). The green fluorescent protein gene was removed from pFP12 by digestion with SphI and StuI restriction endonucleases. The GNA1870 v.1 gene, including the putative FUR box promoter from N. meningitidis strain MC58, was amplified from genomic DNA by polymerase chain reaction (PCR) by use of the following primers: (GNA1870FURSphIF 5′) 5′-ATCGGCATGCGCCGTTCGGACGACATTTG-3′′and (GNA1870FURStuIR 3′) 5′-AAGAAGGCCTTTATTGCTTGGCGGCAAGGC-3′. The PCR product containing the GNA1870 gene was then digested with SphI and StuI restriction endonucleases and was ligated into the pFP12 plasmid that had been digested with SphI and StuI. The resulting plasmid, pFP12-GNA1870 (see figure 1), was transformed and propagated in Escherichia coli strain TOP10 competent cells (Invitrogen). The cells were grown in Luria-Bertani medium at 37°C under chloramphenicol selection (50 μg/mL).
Figure 1.
Construction of pFP12-GNA1870 shuttle vector construct. The green fluorescent protein (GFP) gene was replaced with the GNA1870 gene from Neisseria meningitidis strain MC58 at the SphI/StuI site. cat, chloramphenicol acetyl-transferase; ori, origin of replication.
Overexpression of GNA1870 v.1 protein by N. meningitidis
We prepared a mutant strain of RM1090 in which the GNA1870 v.2 gene was inactivated (RM1090ΔGNA1870) by use of homologous recombination with plasmid pBSUDGNA1870ERM [11] (gift from J. Adu-Bobie, Chiron Vaccines) and erythromycin selection (5 μg/mL). RM1090ΔGNA1870 was transformed with pFP12-GNA1870, as described elsewhere [15], to generate a second-generation mutant that overexpressed GNA1870 v.1 protein. All strains containing the introduced pFP12-GNA1870 shuttle vector were grown in the presence of 5 μg/mL chloramphenicol.
Vaccines
The wild-type (wt) and mutant RM1090 strains were inoculated into Mueller-Hinton broth containing 0.25% glucose and were incubated at 37°C with rocking until the optical density measured at 620 nm reached 0.8–1.0. Phenol was added (0.5% wt/vol), and the broth was left to incubate overnight at 4°C, to kill the bacteria. The bacterial cells were pelleted by centrifugation (at 10,000 g) for 30 min at 4°C and were frozen and stored at −20°C until they were used for preparation of the OMV vaccines. The OMV vaccines were prepared as described elsewhere without the use of detergents, to avoid extraction of the GNA1870 lipoprotein [16]. The rGNA1870 protein vaccine was purified and expressed in E. coli, as described elsewhere [10], by use of a GNA1870 DNA sequence encoding 6 carboxy-terminal histidines and devoid of the amino terminal sequence coding for the putative leader peptide.
Immunization
N. meningitidis OMV preparations and rGNA1870 protein were adsorbed with an equal volume of aluminum phosphate adjuvant (1% Alhydrogel [wt/vol; Superfos Biosector] that had been incubated with PBS to convert aluminum hydroxide to aluminum phosphate). Groups of female CD1 mice, 4–6 weeks old (10 mice/group; Charles River Breeding Laboratories), were immunized intraperitoneally (ip). Each mouse received a dose containing 5 μg of total protein (5 μg of OMV preparations or rGNA1870 protein or 2.5 μg each of OMV preparations and rGNA1870 protein). A total of 3 injections were given, each separated by 3 weeks. Two weeks after the third dose, anesthetized mice were killed by cardiac puncture. Serum samples were separated and were stored frozen at −20°C.
Anti-GNA1870 antibody response
Serum antibody titers against GNA1870 were measured by ELISA, which was performed as described elsewhere [10]. The secondary detecting antibodies were alkaline phosphatase–conjugated goat anti-mouse antiserum specific for IgM, IgG1, IgG2a, IgG2b, and IgG3 (Southern Biotech).
Complement-mediated bactericidal antibody activity
The bactericidal assay was performed as described elsewhere [17], and log phase bacteria grown in Mueller Hinton broth supplemented with 0.25% glucose were used. The final reaction mixture contained 20% (vol/vol) complement and serial 2-fold dilutions of test serum diluted in Gey’s buffer. Bactericidal titers were defined as the serum dilution resulting in a 50% decrease in colony-forming units per milliliter after a 60-min incubation of bacteria in the reaction mixture, compared with the value in controls at time 0. The complement source was human serum from a healthy adult that had no detectable intrinsic bactericidal activity and was qualified as described elsewhere [18].
Binding of antibodies to the surface of live encapsulated N. meningitidis
The ability of anti-GNA1870 antibodies to bind to the surface of live N. meningitidis was determined by flow-cytometric detection of indirect fluorescence, which was performed as described elsewhere [19].
Control antibodies and antiserum
Controls in the different experiments included mouse monoclonal antibodies (MAbs) specific for groups B and C polysaccharide capsules [20, 21]), PorA P1.2 [19], and GNA1870 v.1 protein (JAR3) [10]. In addition, hyperimmune antiserum from mice immunized with rGNA1870 proteins (expressed in E. coli from the genes of N. meningitidis strains MC58, 2996, and M1239 [v.1, v.2, and variant 3 {v.3} proteins, respectively]) and purified as HisTag proteins, as described elsewhere [10, 11], was used.
Activation of human complement deposition on the surface of live encapsulated meningococci
Anti-GNA1870 antibody– dependent deposition of C3b or iC3b on the bacterial surface of live N. meningitidis bacteria was determined by flow cytometry [22].
Passive protection in infant rats
The ability of antiserum to confer passive protection against N. meningitidis group B bacteremia was tested in infant rats challenged ip with group B strain NZ98/254, and this experiment was performed as described elsewhere [17, 22, 23].
RESULTS
Surface accessibility of GNA1870 in N. meningitidis.
To determine whether the GNA1870 protein expressed by strain RM1090 that was transformed with pFP12-GNA1870 is an integral part of the OMV and is surface exposed, we measured binding of anti-GNA1870 antibody to live encapsulated bacterial cells by flow cytometry (figure 2). Positive control MAbs specific for group C capsular polysaccharide (figure 2, column 2) or PorA (anti-P1.2, column 3) showed strong binding to the wt (row B) and 2 mutant RM1090 strains: RM1090ΔGNA1870 (row A) and the RM1090 strain overex-pressing GNA1870 v.1 protein (row C). Compared with cells incubated with the negative control antiserum (column 1), the RM1090 strain overexpressing GNA1870 v.1 protein showed strong binding with both a MAb specific for GNA1870 v.1 protein (column 4) and polyclonal anti-GNA1870 antiserum prepared in mice immunized with v.1, v.2, and v.3 proteins (columns 5 and 6). In contrast, there was no significant binding of these antibodies with the mutant RM1090ΔGNA1870 strain or with the wt RM1090 strain, which naturally expresses low levels of a GNA1870 v.2 protein. Thus, in the RM1090 strain overexpressing GNA1870 v.1 protein, GNA1870 is anchored in the OMV and is surface exposed.
Figure 2.
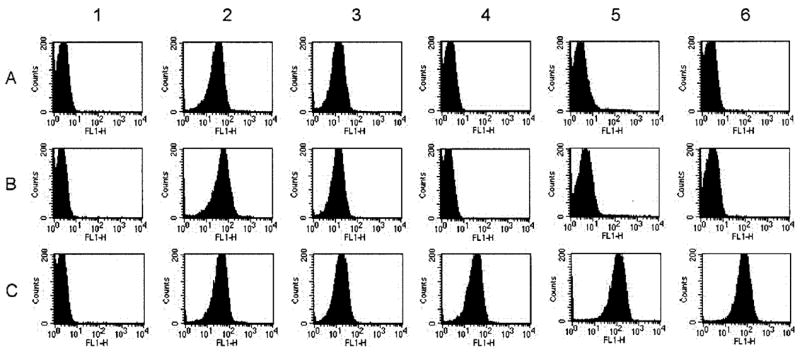
Binding of anti-GNA1870 antibodies with the surface of live encapsulated Neisseria meningitidis cells as determined by indirect fluorescence flow cytometry. A, Strain RM1090ΔGNA1870. B, Wild-type RM1090. C, Strain of RM1090 overexpressing GNA1870 variant 1 (v.1) protein. Column 1, Negative control serum (1:10 dilution) from mice immunized with aluminum phosphate. Column 2, Positive control anti–group C polysaccharide monoclonal antibody (MAb; 10 μg/mL). Column 3, Positive control anti-PorA MAb (1:500 dilution). Column 4, Anti–GNA1870 v.1 protein MAb (2 μg/mL). Column 5, Polyclonal anti-GNA1870 antiserum prepared against v.1, 2, and 3 recombinant proteins (1:10 dilution). Column 6, Same as column 5 but with a 1:250 dilution of serum. FL1-H, relative fluorescence intensity detected using the first channel of the flow cytometer.
OMV vaccines
GNA1870 is expressed in N. meningitidis in low copy numbers [10], and, by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), it resolves in a portion of the gel along with several other proteins. For these reasons, the antigen was not apparent by Coomassie-stained SDS-PAGE, performed as described elsewhere [10, 22], of the different OMV vaccines (~7 μg of total protein/lane). Therefore, we used Western blotting, performed as described elsewhere [10], to evaluate the expression of GNA1870. As is shown in figure 3A, polyclonal mouse antiserum prepared against rGNA1870 v.1, v.2, and v.3 proteins was slightly more reactive with the rGNA1870 v.2 protein than with the v.1 protein. Even with this bias, the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein showed increased reactivity by Western blot, compared with the OMV vaccine prepared from the wt RM1090 strain that naturally expresses a v.2 protein (figure 3B). The results of densitometry measurements indicated that expression of the GNA1870 v.1 protein in the RM1090 strain genetically engineered to overexpress it was ~10-fold higher than that of the v.2 protein expressed naturally by the wt RM1090 strain.
Figure 3.
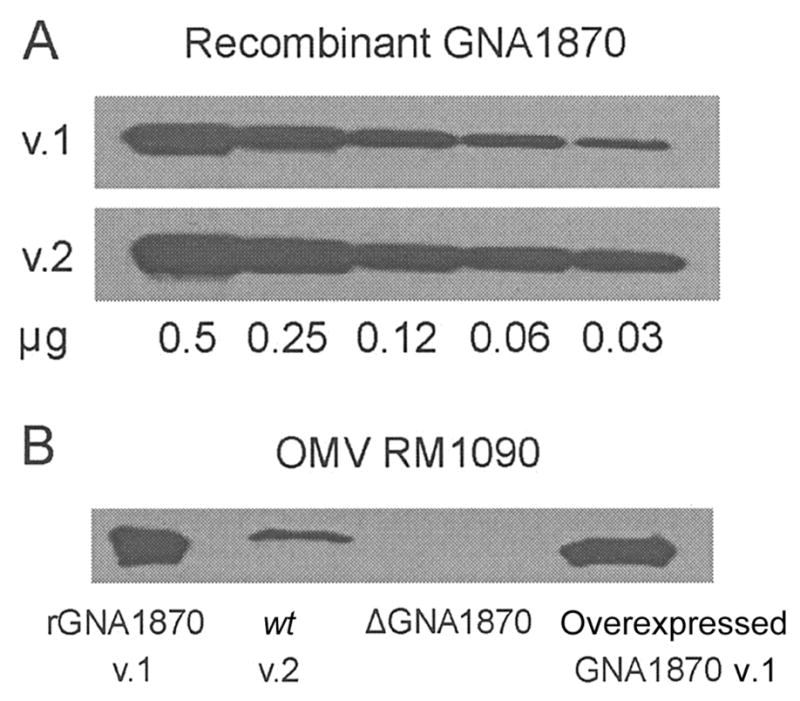
Western blot of outer membrane vesicle (OMV) vaccines. Western blot used polyclonal anti-GNA1870 antiserum from mice immunized with recombinant GNA1870 (rGNA1870) variant 1 (v.1), 2 (v.2), and 3 proteins. A, rGNA1870 v.1 and v.2 proteins. The sensitivity of detection was slightly higher for the rGNA1870 v.2 protein, compared with that for the rGNA1870 v.1 protein. B, rGNA1870 v.1 protein (lane 1), OMV vaccine prepared from wild-type (wt) RM1090 v.2 protein (lane 2), OMV vaccine prepared from strain RM1090ΔGNA1870 (ΔGNA1870; lane 3), and OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein (lane 4). Approximately 5 μg of each protein preparation was used.
Serum anti-GNA1870 antibody responses
Figure 4 summarizes the serum anti-GNA1870 antibody responses of the different groups of mice as measured by ELISA. Control mice immunized with the OMV vaccines prepared from the wt or mutant RM1090ΔGNA1870 strain had negligible anti-GNA1870 antibody responses, compared with those in mice immunized with the rGNA1870 protein vaccine. In contrast, mice immunized with the rGNA1870 protein vaccine only, the mixture of rGNA1870 protein and the OMV vaccine prepared from the RM1090ΔGNA1870 strain, or the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein showed high IgG1 antibody responses and ~10- to 50-fold lower IgG2a and IgG2b responses, respectively. The mice immunized with the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein also showed a modest IgG3 antibody response that was not observed in the other vaccine groups.
Figure 4.
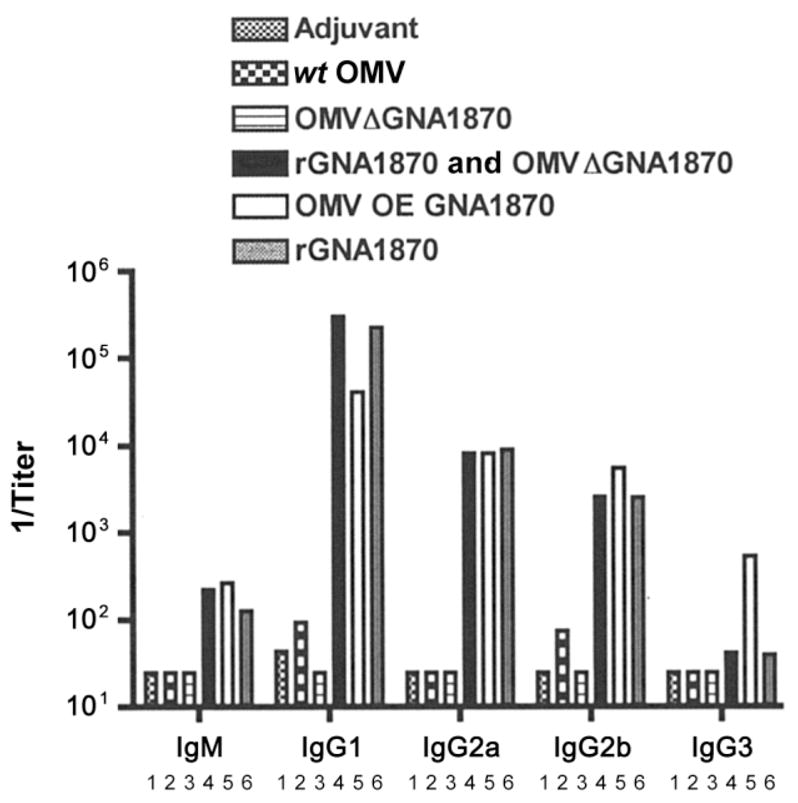
Serum anti-GNA1870 antibody responses as measured by ELISA. The antigen on the plate was recombinant GNA1870 (rGNA1870) variant 1 (v.1) protein. Bar 1, Aluminum phosphate adjuvant. Bar 2, Outer membrane vesicle (OMV) vaccine prepared from wild-type (wt) RM1090. Bar 3, OMV vaccine prepared from strain RM1090ΔGNA1870 (ΔGNA1870). Bar 4, Mixture of OMV vaccine prepared from ΔGNA1870 and rGNA1870 protein. Bar 5, OMV vaccine prepared from RM1090 overexpressing (OE) GNA1870 v.1 protein. Bar 6, rGNA1870 protein vaccine. Each bar represents the geometric mean of the titers measured in 2 serum pools from each vaccine group (each pool contained serum samples from 4–5 mice).
Although the results are not shown in figure 4, we also measured IgG antibody responses to an rGNA1870 v.2 protein (see table 2). Mice immunized with the OMV vaccine prepared from the wt RM1090 strain, which naturally expresses a GNA1870 v.2 protein, showed no detectable anti–GNA1870 v.2 protein antibody responses (geometric mean titer, <1:50), whereas mice immunized with the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein had a cross-reactive anti–GNA1870 v.2 protein geometric mean titer of 1:1200. Thus, in the absence of overexpression of GNA1870 v.1 protein, the OMV vaccine prepared from the wt RM1090 strain elicited negligible IgG antibody responses to the GNA1870 v.2 protein.
Table 2.
Serum IgG anti-GNA1870 antibody responses of mice as measured by ELISA.
| The table is available in its entirety in the online edition of the Journal of Infectious Diseases. |
Serum bactericidal antibody responses
Serum bactericidal activity was measured against 6 genetically diverse encapsulated N. meningitidis strains, 5 of which express subvariants of GNA1870 v.1 proteins (i.e., slight sequence variations from that of the overexpressed GNA1870 protein encoded by the gene from strain MC58 [10, 11]). Mice immunized with the rGNA1870 protein vaccine, the mixture of rGNA1870 protein and the OMV vaccine prepared from the RM1090ΔGNA1870 strain, or the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein developed high bactericidal titers against strain Cu385 that were not significantly different from each other (compare bars 4, 5, and 6 of the upper panel of figure 5). In contrast, there was no detectable serum bactericidal activity in control mice immunized with the OMV vaccines prepared from the wt RM1090 and RM1090ΔGNA1870 strains (bars 2 and 3, respectively; geometric mean titers, <1:10). Cu385 expresses the canonical GNA1870 v.1 protein (which has an amino acid sequence identical to that of strain MC58) and was known on the basis of our previous study [10] to be susceptible to bactericidal activity of antibodies elicited in mice by the rGNA1870 protein vaccine (table 1). Also, Cu385 has a PorA serosubtype (P1.19,15) that is heterologous to that of the wt RM1090 strain (P1.5,2). Therefore, strain Cu385 was expected to be resistant to bactericidal activity of anti-PorA antibodies elicited by the OMV vaccines prepared from the wt RM1090 and RM1090ΔGNA1870 strains [5, 16].
Figure 5.
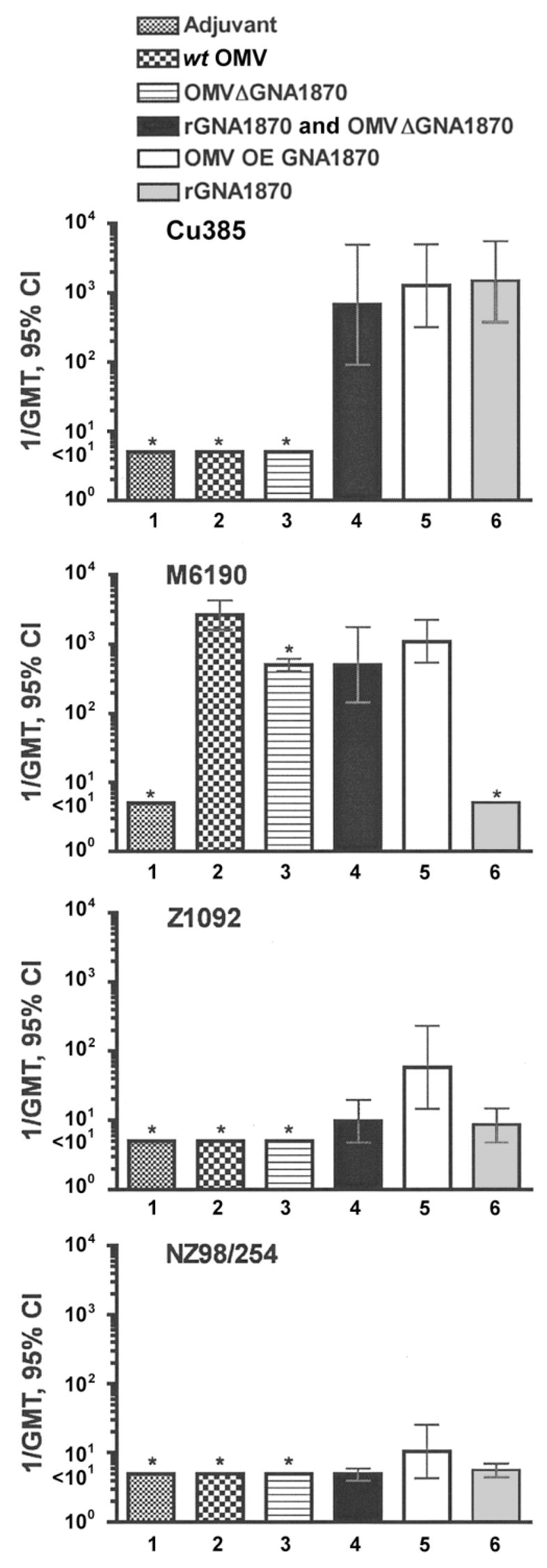
Bactericidal geometric mean titers (GMTs) of the serum samples from mice against 4 representative Neisseria meningitidisstrains. For the vaccine groups, see figure 4. Bars that show the 95% confidence intervals (CIs; whiskers) represent vaccine groups in which serum samples were assayed from 9–10 mice. Bars with asterisks represent geometric means of results from assaying 2 serum pools from each vaccine group (each pool contained serum samples from 4–5 mice).
Figure 5 also shows the corresponding serum bactericidal titers measured against strain M6190, which expresses a sub-variant of GNA1870 v.1 protein, compared with that of the RM1090 strain overexpressing GNA1870 v.1 protein. There was no detectable serum bactericidal activity in mice immunized with the rGNA1870 protein vaccine (bar 6; geometric mean titer, <1:10), a result identical to that of our previous study [10] (table 1). However, serum samples from mice immunized with any of the OMV vaccines were highly bactericidal (bars 2, 3, 4, and 5), which likely reflected bactericidal antibodies against PorA, because the P1.5,2 serosubtype of strain M6190 is homologous with that of the RM1090 vaccine strain.
Figure 5 also shows the bactericidal responses against strains Z1092 and NZ98/254. Both strains express PorA molecules that are heterologous with that of the RM1090 vaccine strain (table 1) and were not killed by serum samples from mice immunized with the OMV vaccines prepared from the wt RM1090 and RM1090ΔGNA1870 strains (geometric mean titers, <1:10). However, mice immunized with the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein (bar 5) had significantly higher bactericidal antibody responses against strain Z1092 than mice immunized with the rGNA1870 protein vaccine (bar 6; P < .02) or mice immunized with the mixture of rGNA1870 protein and the OMV vaccine prepared from the RM1090ΔGNA1870 strain (bar 4; P < .04). Similar trends were observed for the respective serum bactericidal responses measured against strains NZ98/254 (bottom panel of figure 5), BZ198, and M1390 (data not shown). For these 3 strains, serum bactericidal responses were lower than those against strain Z1092, and the titers against strains NZ98/254, BZ198, and M1390 from mice immunized with the vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein were not statistically significantly different from the respective titers of mice in the other vaccine groups (P > .10).
Activation of C3 complement deposition on the surface of live encapsulated N. meningitidis cells
In previous studies, we found that certain antimeningococcal antibodies conferred passive protection against meningococcal bacteremia in the absence of bactericidal activity [10, 22]. Protection was correlated with the ability of the antibodies to activate deposition of iC3b or C3b on the surface of live encapsulated meningococci. Therefore, we investigated the ability of the antiserum from mice immunized with the different OMV vaccines to activate human C3 deposition, as measured by flow cytometry (figure 6). For these experiments, we tested 2 N. meningitidis strains, NZ98/254 (row A) and M1390 (row B), because the serum samples from mice immunized with the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein did not show significantly higher bactericidal titers against these strains than serum samples from mice in the other vaccine groups (see, e.g., the bottom panel of figure 5).
Figure 6.
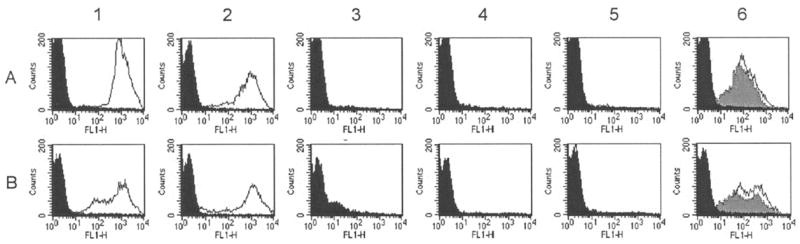
Activation of human C3b and iC3b complement deposition on the surface of live encapsulated Neisseria meningitidis cells as determined by indirect fluorescence flow cytometry. A, Strain NZ98/254. B, Strain M1390. Column 1, Complement plus a positive control group B anticapsular monoclonal antibody (MAb; 25 μg/mL; white area under the curve) or a 1:40 dilution of a serum pool from negative control mice immunized with aluminum phosphate (black area). Column 2, Complement and anti–GNA1870 variant 1 (v.1) protein (JAR3) MAb (1 μg/mL; white area under the curve) or heat-inactivated complement and anti-GNA1870 MAb (5 μg/mL; black area). Columns 3, 4, and 5, Complement and 1:100 dilution of serum pools from mice immunized with recombinant GNA1870 (rGNA1870) protein vaccine (column 3), outer membrane vesicle (OMV) vaccine prepared from wild-type RM1090 (column 4), or rGNA1870 protein mixed with OMV vaccine prepared from strain RM1090ΔGNA1870 (column 5). Column 6, Complement and 1:100 (white area under the curve) and 1:400 dilutions (gray area) of a serum pool from mice immunized with OMV vaccine prepared from RM1090 strain overexpressing GNA1870 v.1 protein. For comparison, panels in column 6 also show data from complement and a 1:100 dilution of a serum pool from mice immunized with OMV vaccine prepared from strain RM1090ΔGNA1870 (black area). FL1-H, relative fluorescence intensity detected using the first channel of the flow cytometer.
There was no evidence of complement deposition when the bacterial cells of either test strain were incubated with the human complement source alone or with complement and a 1: 40 dilution of negative control serum from mice immunized with aluminum phosphate (black areas of panels in column 1). Similarly, there was no detectable C3 deposition with heat-inactivated complement plus 5 μg/mL of a positive control mouse MAb to GNA1870 v.1 protein (JAR 3) (black areas of panels in column 2). In contrast, the addition of active complement to 1 μg/mL anti-GNA1870 MAb (white area under the curve of panels in column 2) or to 25 μg/mL of a positive control group B anticapsular MAb (white area under the curve of panels in column 1) elicited deposition of C3 on the bacterial surface of both test strains, as evidenced by increases in the percentages of bacteria showing strong immunofluorescence.
The panels in columns 3–6 show the effect of adding complement to dilutions of serum pools from groups of mice immunized with different vaccines. The addition of complement to a 1:100 dilution of serum from mice immunized with the rGNA1870 protein vaccine (column 3) or the OMV vaccine prepared from the wt RM1090 strain (column 4) or the OMV vaccine prepared from strain RM1090ΔGNA1870 mixed with rGNA1870 protein (column 5) did not activate C3 deposition. In contrast, dilutions of 1:100 or 1:400 of serum samples from mice immunized with the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein activated strong C3 deposition against both test strains (column 6).
Role of anti-GNA1870 antibody in functional activity
The higher functional activity of the antiserum from mice immunized with the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein could have resulted from antibodies elicited by antigens other than GNA1870. To investigate this possibility, we used an anti-GNA1870 affinity column to absorb a serum pool from mice immunized with the OMV vaccine prepared from the RM1090 strain overex-pressing GNA1870 v.1 protein. By ELISA, 99% of the anti-GNA1870 antibodies were removed, and the absorbed antiserum lost all ability to activate human C3 deposition on N. meningitidis strain NZ98/254 (table 3). In contrast, there was no effect on C3 deposition by absorbing the serum pool on an anti-NadA affinity column, which served as a negative control. Table 3 also summarizes the bactericidal titers of the absorbed serum pools as measured against strains Cu385 and M6190. Absorption of the anti-GNA1870 antibodies completely removed the bactericidal activity against strain Cu385 but had no significant effect on the titers against strain M6190. This latter result was expected, because, as noted above, strain M6190 expresses a PorA with a homologous serosubtype to that of the RM1090 vaccine strain, and the bactericidal anti-PorA antibodies would not be removed by absorption with the GNA1870 or NadA affinity columns. In other experiments, we tested bactericidal activity against a PorA-deficient mutant of strain M6190 that was prepared by selection with complement and anti-PorA P1.2 antibody. The results are summarized in table 4. In contrast to the results with the wt M6190 parent strain, the PorA-deficient mutant was susceptible to complement-mediated bactericidal activity of antibodies elicited by the rGNA1870 protein vaccine (geometric mean titer, 1:1000) or the vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein (geometric mean titer, 1:775) but not by antibodies elicited by the OMV vaccine prepared from the wt RM1090 strain (geometric mean titer, <1:10; see Discussion).
Table 3.
Functional activity of antiserum from mice immunized with outer membrane vesicle (OMV) vaccine prepared from Neisseria meningitidis RM1090 strain overexpressing GNA1870 variant 1 (v.1) protein after depletion of anti-GNA1870 antibodies.
| 1/antibody titer
|
|||
|---|---|---|---|
| Assay | Serum not absorbed | Serum absorbed with GNA1870 | Serum absorbed with NadA |
| Anti-GNA1870 ELISA | 40,000 | 400 | 30,000 |
| C3b complement deposition,a strain NZ98/254 | ≥400 | <25 | ≥400 |
| Bactericidal activity | |||
| Strain Cu385 | 2500 | <10 | 3000 |
| Strain M6190 | 1000 | 600 | 600 |
NOTE. A serum pool was prepared from mice immunized with OMV vaccine prepared from the RM1090 strain over-expressing GNA1870 v.1 protein. The antiserum was absorbed on a column containing recombinant GNA1870-HisTag protein or, as a negative control, recombinant NadA-HisTag protein [37], both of which were complexed with Ni-NTA Sepharose (Qiagen). The respective pass-through fractions were combined and concentrated to their original serum volume by membrane filtration.
Serum dilution in the flow-cytometric complement activation assay that elicited a 10-fold increase in immunofluorescence, compared with that for the negative control serum (see, e.g., figure 5).
Table 4.
Decreased expression of PorA in Neisseria meningitidis strain M6190 is associated with increased susceptibility to the bactericidal activity of anti-GNA1870 antibody.
| 1/bactericidal titera |
||
|---|---|---|
| Antibody | Wt strain | PorA-deficient strainb |
| Anticapsular MAb (SEAM 12) | 60 | 50 |
| Anti–PorA P1.2 MAb | 700 | <50 |
| Mouse polyclonal antiserum | ||
| Aluminum phosphate (negative control) | <10 | <10 |
| OMV from wt RM1090 | 500 | <10 |
| OMV from RM1090 strain overexpressing GNA1870 v.1 protein | 375 | 775 |
| Recombinant GNA1870 v.1 protein | <10 | 1000 |
Passive protection in the infant rat meningococcal bacteremia model
Infant rats were pretreated with 1:15 dilutions of serum pools from the different groups of mice and were challenged 2 h later with N. meningitidis strain NZ98/254. Figure 7 shows the geometric mean colony-forming units per milliliter in blood obtained ~6 h after the challenge. All 10 rats treated with the serum pool from negative control mice immunized with aluminum phosphate had bacteremia with a geometric mean of ~105 cfu/mL (bar 1). In contrast, pretreatment with 10 μg/rat of a positive control group B anticapsular MAb (bar 2) or 10 μg/rat of an anti–rGNA1870 protein MAb (bar 3) resulted in a geometric mean that was 3–4 log10 cfu/mL lower (P < .0001). In contrast, there was no significant protective activity by serum pools from mice immunized with the OMV vaccine prepared from the RM1090ΔGNA1870 strain (bar 4) or the OMV vaccine prepared from the RM1090ΔGNA1870 strain mixed with rGNA1870 protein (bar 5). However, the serum pool from mice immunized with the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein (bar 6) conferred protection (4 log10 cfu/mL decrease in the geometric mean, compared with negative control rats; P < .0001). The serum pool from mice immunized with the rGNA1870 protein vaccine (bar 7) also conferred protection (~2 log10 cfu/mL decrease in the geometric mean; P < .0001) but was less protective than the serum pool from mice immunized with the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein (P < .0001).
Figure 7.
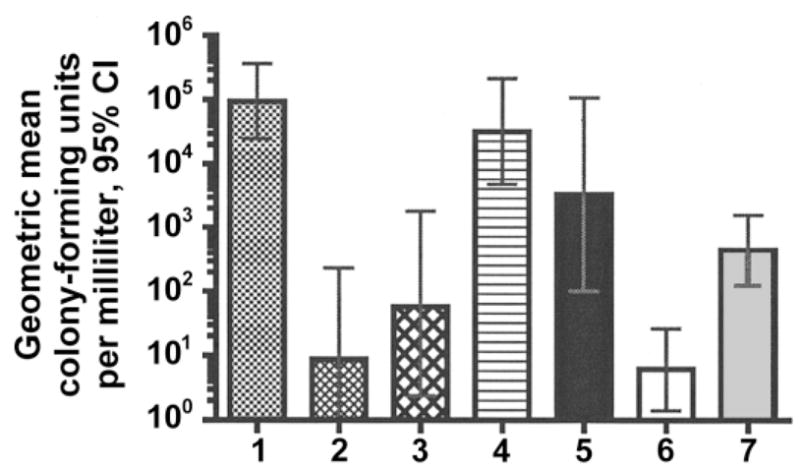
Passive protection in the infant rat meningococcal bacteremia model. Ten rats per group were treated intraperitoneally (ip) with 100 μL of different serum pools diluted 1:15 and were challenged ip 2 h later with ~60,000 cfu of group B strain NZ98/254. Quantitative blood cultures were obtained 4–6 h later, and the geometric mean colony-forming units per milliliter were calculated. Bar 1, Serum from mice immunized with aluminum phosphate. Bar 2, Anticapsular monoclonal antibody (MAb; 10 μg/rat). Bar 3, Anti-GNA1870 MAb (10 μg/rat). Bar 4, outer membrane vesicle (OMV) vaccine prepared from RM1090ΔGNA1870. Bar 5, OMV vaccine prepared from RM1090ΔGNA mixed with recombinant GNA1870 (rGNA1870) protein. Bar 6, OMV vaccine prepared from RM1090 overexpressing GNA1870 variant 1 protein. Bar 7, rGNA1870 protein vaccine. CI, confidence interval.
DISCUSSION
In previous studies, mice immunized with a rGNA1870 protein vaccine developed high serum bactericidal antibody responses against most strains expressing GNA1870 proteins of the homologous variant group [10, 11]. However, a number of strains that expressed subvariants of the respective GNA1870 protein were resistant to anti-GNA1870 complement-mediated bactericidal activity.
In an effort to enhance protective activity, we investigated the immunogenicity of an OMV vaccine prepared from a N. meningitidis strain that was genetically engineered to overex-press GNA1870 v.1 protein. The serum anti-GNA1870 antibodies elicited by this modified GNA1870-OMV vaccine had a greater bactericidal response against N. meningitidis strains expressing subvariants of the GNA1870 v.1 protein than those elicited by the rGNA1870 protein vaccine or the OMV vaccine prepared from the wt RM1090 strain or a mixture of rGNA1870 protein and the OMV vaccine prepared from the wt RM1090 strain. The antibodies elicited by the OMV vaccine prepared from the RM1090 strain overexpressing GNA1870 v.1 protein also gave greater C3 deposition on the surface of strains NZ98/254 and M1390 (figure 6, column 6) and greater passive protection against bacteremia in infant rats challenged with strain NZ98/254 (figure 7). The ability of antibodies to confer passive protection in the absence of bactericidal activity in an animal model has been reported elsewhere [10, 22], and it likely results from opsonization [24, 25].
O’Dwyer et al. [26] recently described the results of immunizing mice with an OMV vaccine prepared from a commensal N. flavescens strain engineered to express another conserved meningococcal vaccine candidate, NspA. N. flavescens does not naturally express PorA or NspA. The immunized mice developed NspA-specific serum opsonophagocytic activity. After absorption of antibodies against wt N. flavescens OMV vaccine, the residual anti-NspA antibodies conferred passive protection to mice given a lethal challenge of an encapsulated N. meningitidis strain. However, in contrast to the results with the modified GNA1870-OMV vaccine investigated in the present study, the recombinant NspA–N. flavescens OMV vaccine did not elicit serum bactericidal antibody responses.
Defining the mechanisms by which the modified GNA1870-OMV vaccine elicits serum antibodies that have broader functional activity than those elicited by the rGNA1870 protein vaccine will require further study. OMV vaccines are complex mixtures of antigens and would be expected to elicit antibodies directed against a number of antigenic targets. Our serum absorption studies demonstrated that the ability of the OMV vaccine prepared from RM1090 overexpressing GNA1870 v.1 protein to elicit serum bactericidal antibodies to strain Cu385 and to activate C3 deposition on strain NZ98/254 was a result of anti-GNA1870 antibodies (table 3). Recently, the region of GNA1870 comprising aa 100–254 has been shown to be important in eliciting bactericidal responses [27]. Conceivably, expression of the native GNA1870 protein anchored within the OMV permitted better expression of certain conformational epitopes in this region than in the corresponding portion of the nonlipidated recombinant protein vaccine.
An important second property of the modified GNA1870-OMV vaccine is its ability to elicit serosubtype-specific bactericidal activity against PorA. This result was expected on the basis of results from previous studies that identified PorA as an important antigenic target of antibodies elicited by OMV vaccines [5, 28] and was confirmed in the present study by the lack of bactericidal activity in serum samples from mice immunized with a conventional OMV vaccine when they were tested against a mutant strain of M6190 deficient in PorA expression (table 4). Unexpectedly, the PorA-deficient mutant strain was susceptible to bactericidal activity by antibodies elicited by the rGNA1870 protein, whereas the parent PorA-sufficient wt strain was resistant (table 4). By flow cytometry and Western blot, the encapsulated PorA-deficient mutant strain had more surface-accessible GNA1870 than the PorA-sufficient wt strain (authors’ unpublished data), which likely accounted for the susceptibility of the PorA-deficient mutant strain to bactericidal activity by anti-GNA1870 antibodies. Thus, another unanticipated advantage of the modified GNA1870-OMV vaccine over a conventional OMV vaccine is the ability of the former to decrease the likelihood of immune selection of PorA-deficient escape mutants [29–33], which can decrease the susceptibility of strains to the bactericidal activity of anti-PorA antibodies [34].
Acknowledgments
We thank Zyde Raad, Patricia Zuno-Mitchell, and Maggie Ching, for excellent technical assistance; Gregory Moe and Alexander Lucas (Children’s Hospital Oakland Research Institute), for invaluable critical comments on the manuscript; and Jo-Anne Dillon (University of Saskatchewan, Saskatoon, Saskatchewan, Canada), for the pFP12 shuttle vector used for the expression of GNA1870.
Financial support: National Institute of Allergy and Infectious Diseases (public health service grants RO1 AI46464 and R21 AI061533); National Institutes of Health (training grant T32-HL007951 to J.A.W.).
Footnotes
Potential conflicts of interest: D.M.G. is a part-time consultant for Chiron Vaccines and Sanofi Pasteur.
References
- 1.Jodar L, Feavers I, Salisbury D, Granoff DM. Development of vaccines against meningococcal disease. Lancet. 2002;359:1499–508. doi: 10.1016/S0140-6736(02)08416-7. [DOI] [PubMed] [Google Scholar]
- 2.Thomas M. Prevention of group B meningococcal disease by vaccination: a difficult task. N Z Med J. 2004;117:U1016. [PubMed] [Google Scholar]
- 3.Desmond N. Getting to grips with an epidemic. Nurs N Z. 2004;10:2. [PubMed] [Google Scholar]
- 4.Baker MG, Martin DR, Kieft CE, Lennon D. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991–2000. J Paediatr Child Health. 2001;37:S13–9. doi: 10.1046/j.1440-1754.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- 5.Tappero JW, Lagos R, Ballesteros AM, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA. 1999;281:1520–7. doi: 10.1001/jama.281.16.1520. [DOI] [PubMed] [Google Scholar]
- 6.Sacchi CT, Whitney AM, Popovic T, et al. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States 1992–1998. J Infect Dis. 2000;182:1169–76. doi: 10.1086/315833. [DOI] [PubMed] [Google Scholar]
- 7.Martin D, Cadieux N, Hamel J, Brodeur BR. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J Exp Med. 1997;185:1173–83. doi: 10.1084/jem.185.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou VC, Moe GR, Raad Z, Wuorimaa T, Granoff DM. Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies. Infect Immun. 2003;71:6844–9. doi: 10.1128/IAI.71.12.6844-6849.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizza M, Scarlato V, Masignani V, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–20. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 10.Welsch JA, Rossi R, Comanducci M, Granoff DM. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol. 2004;172:5606–15. doi: 10.4049/jimmunol.172.9.5606. [DOI] [PubMed] [Google Scholar]
- 11.Masignani V, Comanducci M, Giuliani MM, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–99. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caugant DA, Bovre K, Gaustad P, et al. Multilocus genotypes determined by enzyme electrophoresis of Neisseria meningitidis isolated from patients with systemic disease and from healthy carriers. J Gen Microbiol. 1986;132:641–52. doi: 10.1099/00221287-132-3-641. [DOI] [PubMed] [Google Scholar]
- 13.Caugant DA, Bol P, Hoiby EA, Zanen HC, Froholm LO. Clones of serogroup B Neisseria meningitidis causing systemic disease in The Netherlands, 1958–1986. J Infect Dis. 1990;162:867–74. doi: 10.1093/infdis/162.4.867. [DOI] [PubMed] [Google Scholar]
- 14.Maiden MC, Bygraves JA, Feil E, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–5. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagotto FJ, Salimnia H, Totten PA, Dillon JR. Stable shuttle vectors for Neisseria gonorrhoeae, Haemophilus spp. and other bacteria based on a single origin of replication. Gene. 2000;244:13–9. doi: 10.1016/s0378-1119(99)00557-0. [DOI] [PubMed] [Google Scholar]
- 16.Moe GR, Zuno-Mitchell P, Hammond SN, Granoff DM. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect Immun. 2002;70:6021–31. doi: 10.1128/IAI.70.11.6021-6031.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moe GR, Tan S, Granoff DM. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect Immun. 1999;67:5664–75. doi: 10.1128/iai.67.11.5664-5675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos GF, Deck RR, Donnelly J, Blackwelder W, Granoff DM. Importance of complement source in measuring meningococcal bactericidal titers. Clin Diagn Lab Immunol. 2001;8:616–23. doi: 10.1128/CDLI.8.3.616-623.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granoff DM, Moe GR, Giuliani MM, et al. A novel mimetic antigen eliciting protective antibody to Neisseria meningitidis. J Immunol. 2001;167:6487–96. doi: 10.4049/jimmunol.167.11.6487. [DOI] [PubMed] [Google Scholar]
- 20.Granoff DM, Bartoloni A, Ricci S, et al. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J Immunol. 1998;160:5028–36. [PubMed] [Google Scholar]
- 21.Garcia-Ojeda PA, Monser ME, Rubinstein LJ, Jennings HJ, Stein KE. Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent toxoid conjugate vaccine. Infect Immun. 2000;68:239–46. doi: 10.1128/iai.68.1.239-246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsch JA, Moe GR, Rossi R, Adu-Bobie J, Rappuoli R, Granoff DM. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis. 2003;188:1730–40. doi: 10.1086/379375. [DOI] [PubMed] [Google Scholar]
- 23.Moe GR, Zuno-Mitchell P, Lee SS, Lucas AH, Granoff DM. Functional activity of anti-neisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect Immun. 2001;69:3762–71. doi: 10.1128/IAI.69.6.3762-3771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmenate T, Mesa C, Menendez T, Falcon V, Musacchio A. Recombinant Opc protein from Neisseria meningitidis reconstituted into liposomes elicits opsonic antibodies following immunization. Biotechnol Appl Biochem. 2001;34:63–9. doi: 10.1042/ba20010008. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann AK, Gorringe AR, Reddin KM, West K, Smith I, Halstensen A. Human opsonins induced during meningococcal disease recognize transferrin binding protein complexes. Infect Immun. 1999;67:6526–32. doi: 10.1128/iai.67.12.6526-6532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Dwyer CA, Reddin K, Martin D, et al. Expression of heterologous antigens in commensal Neisseria spp.: preservation of conformational epitopes with vaccine potential. Infect Immun. 2004;72:6511–8. doi: 10.1128/IAI.72.11.6511-6518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuliani MM, Santini L, Brunelli B, et al. The region comprising amino acids 100 to 255 of Neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infect Immun. 2005;73:1151–60. doi: 10.1128/IAI.73.2.1151-1160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milagres LG, Gorla MC, Sacchi CT, Rodrigues MM. Specificity of bactericidal antibody response to serogroup B meningococcal strains in Brazilian children after immunization with an outer membrane vaccine. Infect Immun. 1998;66:4755–61. doi: 10.1128/iai.66.10.4755-4761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Ende A, Hopman CT, Keijzers WC, et al. Outbreak of meningococcal disease caused by PorA-deficient meningococci. J Infect Dis. 2003;187:869–71. doi: 10.1086/367899. [DOI] [PubMed] [Google Scholar]
- 30.van der Ende A, Hopman CT, Zaat S, Essink BB, Berkhout B, Dankert J. Variable expression of class 1 outer membrane protein in Neisseria meningitidis is caused by variation in the spacing between the −10 and −35 regions of the promoter. J Bacteriol. 1995;177:2475–80. doi: 10.1128/jb.177.9.2475-2480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Ende A, Hopman CT, Dankert J. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect Immun. 2000;68:6685–90. doi: 10.1128/iai.68.12.6685-6690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jelfs J, Munro R, Wedege E, Caugant DA. Sequence variation in the porA gene of a clone of Neisseria meningitidis during epidemic spread. Clin Diagn Lab Immunol. 2000;7:390–5. doi: 10.1128/cdli.7.3.390-395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcala B, Salcedo C, Arreaza L, et al. Antigenic and/or phase variation of PorA protein in non-subtypable Neisseria meningitidis strains isolated in Spain. J Med Microbiol. 2004;53:515–8. doi: 10.1099/jmm.0.05517-0. [DOI] [PubMed] [Google Scholar]
- 34.Martin SL, Borrow R, van der Ley P, Dawson M, Fox AJ, Cartwright KA. Effect of sequence variation in meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine. Vaccine. 2000;18:2476–81. doi: 10.1016/s0264-410x(00)00047-5. [DOI] [PubMed] [Google Scholar]
- 35.Russell JE, Jolley KA, Feavers IM, Maiden MC, Suker J. PorA variable regions of Neisseria meningitidis. Emerg Infect Dis. 2004;10:674–8. doi: 10.3201/eid1004.030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacchi CT, Lemos AP, Brandt ME, et al. Proposed standardization of Neisseria meningitidis PorA variable-region typing nomenclature. Clin Diagn Lab Immunol. 1998;5:845–55. doi: 10.1128/cdli.5.6.845-855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comanducci M, Bambini S, Brunelli B, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002;195:1445–54. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]