SPECIFIC AIMS
We recently reported that sulforaphane (SFN), an isothiocyanate from broccoli, inhibited HDAC activity and increased the acetylation of histone H3 and histone H4 in human colon cancer cells. Subsequent work in prostate cancer cells provided evidence for: 1) inhibition of HDAC; 2) increased histone acetylation, globally, as well as locally in association with P21 and bax promoters; 3) increased p21 and Bax mRNA and protein expression; and 4) activation of caspases. The aim of the present study was to examine the effects of SFN on HDAC in vivo, and investigate suppression of intestinal polyps in the Apcmin mouse.
PRINCIPAL FINDINGS
1. SFN and SFN-N-acetylcysteine (SFN-NAC) inhibit HDAC activity in mouse colonic mucosa
An initial comparison of SFN with one of its metabolites—namely, SFN-NAC—showed that both compounds inhibited HDAC activity significantly in mouse colonic mucosa, 6 h after single oral gavage treatment (Fig. 1A). HDAC activity was decreased by 50% with SFN-NAC and by ~65% with SFN (P<0.001 vs. controls). Acetylated histones corresponded well with HDAC inhibition (Fig. 1B), such that SFN and SFN-NAC each produced an increase in acetylated histone H3 and acetylated histone H4 in the colon, compared with controls given vehicle alone. A time course study with SFN showed that acetylated his-tones were increased ~2-fold after 6 and 24 h and declined to control levels by 48 h; p21, a known target of HDAC inhibitors, was increased 100% over controls during the 24 – 48 h period (Fig. 2, online version).
Figure 1.
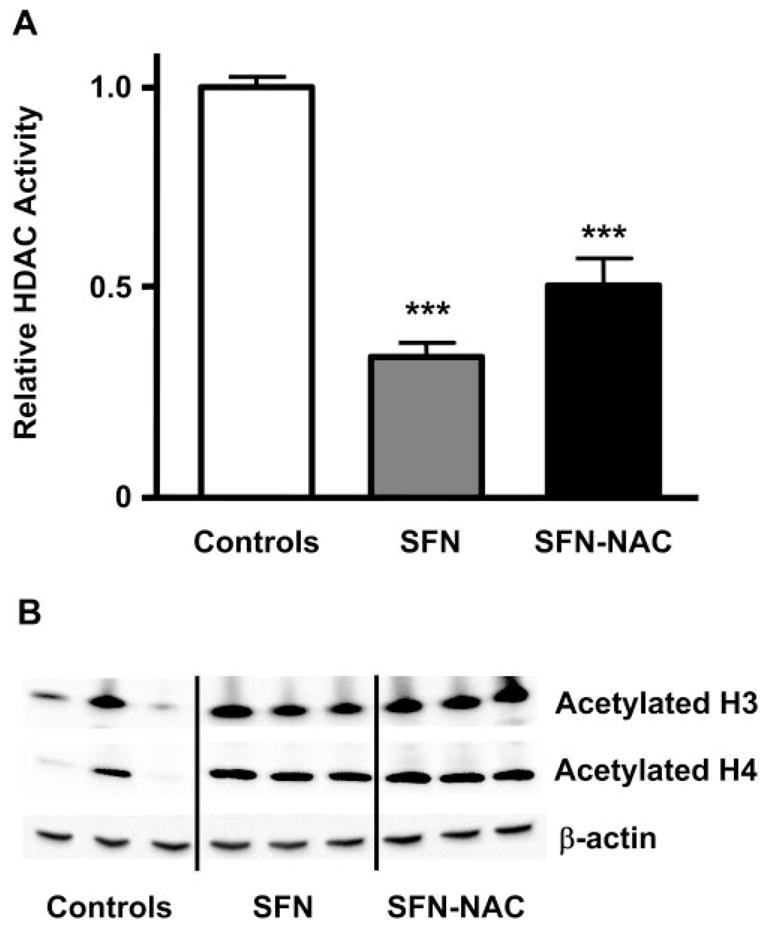
SFN and SFN-NAC inhibit HDAC activity and induce accumulation of acetylated histones in mouse colonic mucosa. Mice were treated by gavage with 10 μmol SFN, 10 μmol SFN-NAC, or vehicle alone, and colonic mucosa was isolated after 6 h. A) HDAC activities, expressed as mean ± SE, n = 3; ***P < 0.001. B) Colonic mucosa from each mouse was analyzed by immunoblot for acetylated histone H3 and acetylated histone H4; β-actin was used as a loading control.
Figure 2.
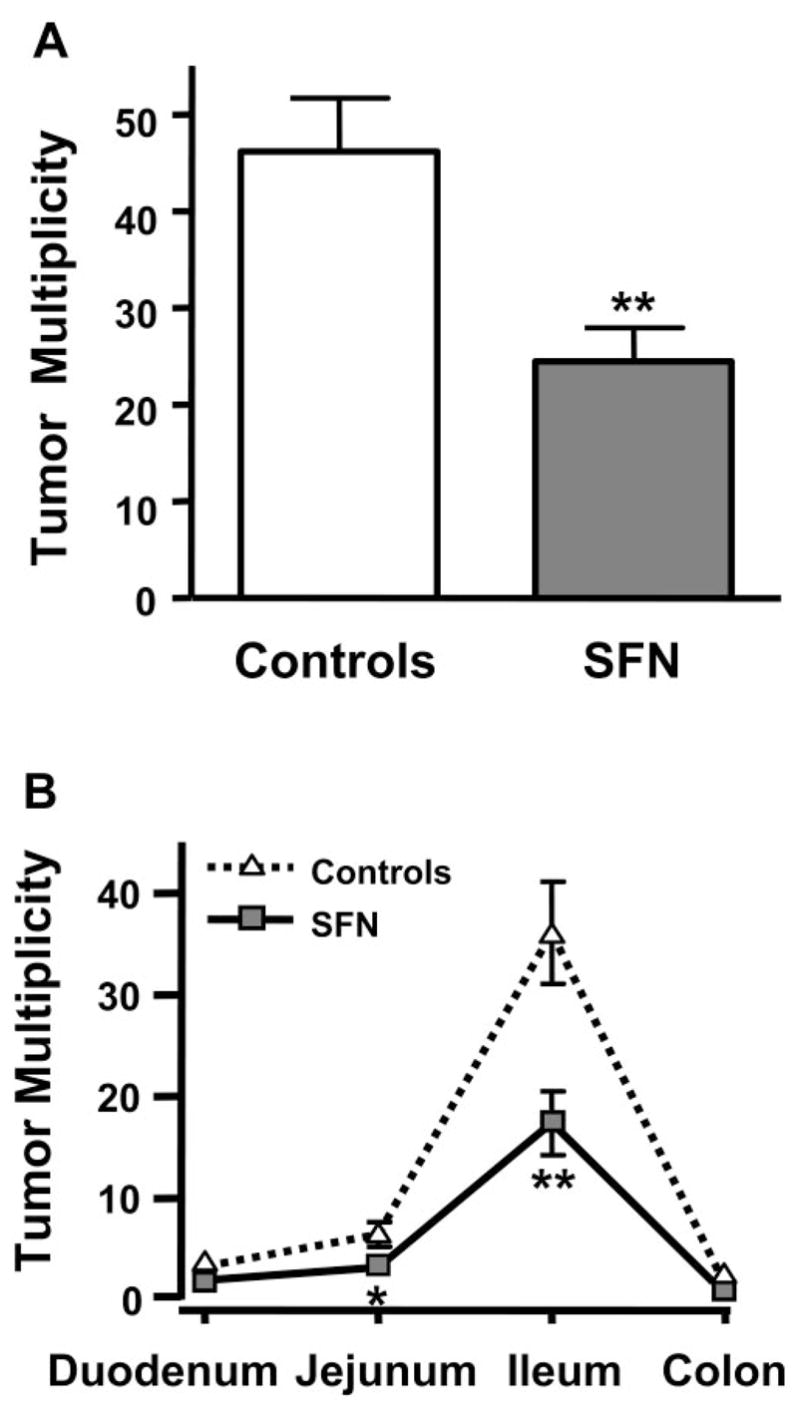
Dietary SFN suppresses polyp formation in Apcmin mice. Mice consumed ~6 μmol SFN/day for 10 wk in the diet or AIN93G diet alone (controls). A) Suppression of overall tumor multiplicity; B) Suppression according to tumor distribution in the GI tract. Mean ± SE, n = 15; *P < 0.05; **P < 0.01.
2. Dietary SFN induces histone acetylation in mouse tissues
Subsequent experiments examined changes in histone acetylation status after administration of SFN in the diet. Mice consumed ~6 μmol SFN/day for 10 wk, with no adverse affect on body weight, hematocrit, or spleen weight (Table 1, online version).
Immunoblot analysis performed on tissues from SFN-fed mice revealed a trend toward increased acetylated histone H3, acetylated H4, and p21 expression in the ileum, colon, peripheral blood mononuclear cells, and prostate (Fig. 3, online version). In prostate tissues there was significant inhibition of HDAC activity by SFN (P<0.01; Fig. 3E, online version); this provides support for a systemic effect of dietary SFN on HDAC, and suggests the need for further studies in prostate cancer models in vivo. Other systemic tissues from SFN-treated mice are currently under investigation for changes in HDAC activity.
Figure 3.
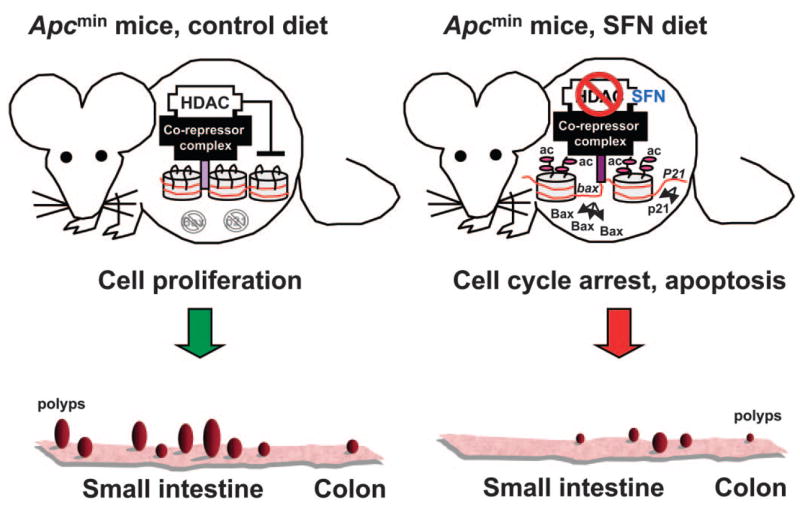
Summary of key findings. In the Apcmin mouse, HDAC/co-repressor complexes maintain a tightly restricted chromatin configuration, which limits access of transcription factors to DNA, and represses genes required for cell cycle checkpoint control and apoptosis. Inhibition of HDAC by SFN enables acetyl groups (“ac”) to be added to histone tails, loosening DNA/chromatin interactions, and allowing access of transcription factors to the promoters of genes such as P21 and bax. Re-expression of these genes triggers cell cycle arrest and apoptosis in transformed cells and microadenomas, thereby suppressing polyp formation.
3. Dietary SFN suppresses polyps in Apcmin mice
The Apcmin mouse develops polyps spontaneously in the small intestine, and occasionally in the colon, and has been used extensively in studies with chemo-preventive agents. In Apcmin mice, SFN suppressed tumor multiplicity significantly compared with animals fed control diet (P<0.01, Fig. 2A; and Fig. 4A, online version). The average tumor yield was lowered by ~50% in all regions of the intestine, but the most significant inhibition was detected in the ileum (P<0.01, Fig. 2B).
4. Dietary SFN induces global histone acetylation in Apcmin mice, as well as increasing histone acetylation associated with P21 and bax gene promoters
In the ileum polyps and adjacent normal looking mucosa of Apcmin mice, SFN produced an increase in acetylated histone H3 expression, and acetylated histone H4 also was elevated in some of the mice (Fig. 5A, online version). In the colon polyps examined, acetylated histone H3 and acetylated histone H4 levels were augmented after SFN treatment, compared with colon polyps obtained from mice on control diet (Fig. 5B, online version).
HDAC inhibitors trigger cell cycle arrest and apoptosis in cancer cells by de-repressing genes such as P21 and bax, but these genes can be modulated by other mechanisms. Thus, we examined the direct association between acetylated histones and target genes of interest using in vivo chromatin immunoprecipitation (ChIP) assays, starting with antibody to acetylated histone H3 and primers specific for the promoter region of P21. Low or undetectable signals were obtained when the ChIP assay was performed on mucosa scraped from the ileum and colon of Apcmin mice fed control diet, but in mice given SFN, there was a marked increase in acetylated histone H3 associated with the promoter region of the P21 gene (Fig. 6A, online version). Similar results were obtained using bax primers in the ChIP assay (Fig. 6B, online version), and immunoblot analysis confirmed a significant increase in Bax expression in the ileum polyps from mice fed SFN, compared with controls (P<0.05, Fig. 6C, online version).
CONCLUSIONS AND SIGNIFIGANCE
SFN was first identified as a potent inducer of Phase 2 enzymes, and this provided the impetus for subsequent studies on the various detoxification pathways involved in carcinogen excretion. SFN also suppressed skin tumors in mice and colonic aberrant crypts in rats when given postinitiation (i.e. solely during the period following carcinogen exposure). We obtained evidence for inhibition of HDAC activity in colon and prostate cancer cells and biochemical studies suggested that the ‘ultimate’ HDAC inhibitor was likely to be SFN-cysteine (SFN-Cys), a metabolite of SFN generated via the mercapturic acid pathway.
In the present investigation, HDAC activity was inhibited significantly in the colonic mucosa of mice after a single oral dose of SFN and SFN-NAC, and there was an increase in acetylated histones as early as 6 h after treatment. These findings provide evidence, for the first time in vivo, of HDAC inhibition by SFN and by one of its metabolites. Moreover, SFN protected significantly against polyp formation in Apcmin mice, with a concomitant increase in global histone acetylation in the tumors. In vivo ChIP assays confirmed an increase in histone acetylation associated with the promoters of P21 and bax.
A key question concerns the conditions under which HDAC inhibition might be most important, in the context of other proposed mechanisms of SFN action, such as checkpoint 2 kinase activation, inhibition of tubulin polymerization, and Keap1-Nrf2 signaling. In (ARE) one scenario, to access the antioxidant response element and to fully activate gene expression, HDACs must be displaced and/or inhibited to allow recruitment of Nrf2 and its co-activators (Fig. 7, online version). It would be instructive to test other known ARE-inducing agents for their ability to inhibit HDAC activity. Use of Nrf2−/− mice implicated Nrf2 as an important player in ARE-driven gene expression by oltipraz, a compound that lacks the structural features of a typical HDAC inhibitor. SFN also was much less effective in Nrf2−/− mice compared with wild-type mice when administered during the period of carcinogen treatment. However, it would be interesting to use Nrf2−/− mice to determine whether chronic dietary SFN administration, postinitiation, results in tumor suppression and HDAC inhibition in tissues such as the colon. Based on the present investigation using wild-type and Apcmin mice (Fig. 3), we propose that HDAC inhibition by SFN induces acetylated histones on the promoters of genes such as P21 and bax, and the derepressed genes trigger cell cycle arrest and apoptosis in transformed cells and microadenomas, thereby suppressing polyp formation in the GI tract.


