Abstract
The purine analogs, fludarabine and cladribine represent an important class of chemotherapy agents used to treat a broad spectrum of lymphoid malignancies. Their toxicity profiles include dose-limiting myelosuppression, immunosuppression, opportunistic infection and severe neurotoxicity. This review summarizes the neurotoxicity of high- and standard-dose fludarabine, focusing on the clinical and pathological manifestations in the eye. The mechanisms of ocular toxicity are probably multifactorial. With increasing clinical use, an awareness of the neurological and ocular vulnerability, particularly to fludarabine, is important owing to the potential for life- and sight-threatening consequences.
Keywords: fludarabine, neurotoxicity, ocular toxicity, ophthalmic pathology, pharmacogenetics, purine analog
The two purine analogs fludarabine and cladribine constitute a major group of anti-metabolite cytotoxic drugs widely used in clinical practice. They are mainly used as agents in a broad spectrum of indolent lymphoid malignancies, including chronic lymphocytic leukemia (CLL), non-Hodgkin’s lymphoma (NHL), Waldenström’s macroglobulinemia (WM), hairy cell leukemia and cutaneous T-cell lymphoma. Other applications use fludarabine to suppress immunological function, for example in facilitating non-myeloablative stem cell transplantation [1]. Recently, fludarabine has also been used with the novel ribonucleotide reductase inhibitor, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, in adults with refractory acute leukemias and aggressive myeloproliferative disorders [2].
The purine analogs share structural similarities and their toxicity profiles include doselimiting myelosuppression, immunosuppression, neutropenic fevers and opportunistic infections, pulmonary toxicity and severe neurotoxicity; however, this last complication has primarily occurred at significantly higher doses than those currently recommended for clinical use. This review summarizes the neurotoxicity of fludarabine, focusing on the ocular manifestations and the toxicities associated with the combination of fludarabine with other medications, specifically other chemotherapeutic agents.
Fludarabine
Preclinical studies on fludarabine
Fludarabine is an antineoplastic agent, which has been studied in patients with a variety of lymphoproliferative malignancies. Alternative names for this compound include 9-d-arabinofuranosyl-2-fluoroadenine 5′-monophosphate 2-fluoro-ara-AMP, and NSC 312887. It was originally synthesized by Montgomery and Hewson in 1969 [3]. Fludarabine has multiple mechanisms of action, most of which are directed toward disruption of DNA synthesis [4-6].
After preclinical experiments in a number of tumor cell lines, including HeLa cells [7], lymphoma cells and animal tumor systems, including L1210 leukemia [8] and P388 leukemia in mice [9] and dogs [10], this agent was approved for clinical trials in 1982 for the treatment of acute monocytic leukemia (AML) [11]. In an animal experiment, dogs were treated at doses of up to fourtimes the mouse equivalent lethal dose (LD)10 on either a single dose or a 5-day schedule, with no neurotoxicity detected [10]. No ocular toxicity was reported in any of these experiments.
Clinical dosage & pharmacokinetics of fludarabine
The pharmacokinetic properties of intravenously administered fludarabine are well established. During Phase I clinical investigation, the pharmacokinetics of high doses of fludarabine phosphate in low-grade lymphoproliferative malignancies, including CLL and NHL patients, were studied [12]. Following bolus intravenous rapid infusion (over 2-5 min) of doses ranging from 80-260 mg/m2, fludarabine is in minutes almost quantitatively converted to cytotoxic F-ara-ATP within the plasma. It is then eliminated in three exponential phases [12]. Plasma concentrations were computer fitted to a three-compartment open model with sequential halflifes of t1/2 α = 4.97 min, t1/2 β = 1.38 h, and t1/2 γ = 10.41 h, with the mean residence time calculated at 10.51 h [12]. Pharmacokinetic analysis of F-ara-A demonstrated a volume of distribution of 96.2 ± 26.0 l/m2 with no plasma accumulation over several days and a mean clearance from the plasma of 9.07 ± 3.77 l/h·m2 [13]. The main route of elimination is renal, with 40-60% of the intravenously administered dose excreted in the urine [12,14]. There appears to be a correlation between creatinine clearance and total body clearance of F-ara-A, necessitating special consideration in patients with impaired renal function.
The recommended intravenous dosage regimen for fludarabine in the treatment of CLL is 25 mg/m2 once daily for 5 consecutive days, with the cycle repeated every 28 days until a maximal response is seen. The response usually requires six cycles of fludarabine therapy. The pharmacokinetics of the standard dose of fludarabine have also been studied. Plasma concentrations of approximately 3 μmol/l F-ara-A are achieved at the end of each infusion [6]. Intracellular levels of the cytotoxic moiety F-ara-ATP peak within 3-4 h of termination of fludarabine infusion and decline monophasically with a median half-life of 23 h [15].
Although used primarily in its intravenous form, fludarabine is now available in a 10 mg immediate-release tablet. After administration of a single oral dose of 50-90 mg of fludarabine to patients with various types of NHL, area under curve (AUC; 0-24 h) and Cmax of F-ara-A were linear and dose proportional. Cmax values were approximately 20-30% of those achieved by intravenous infusion and were reached in 1-2 h. Tmax (the time to Cmax) was independent of the dosage [16]. The bioavailability of oral fludarabine after single or multiple doses is 50-65% [16]; it appears to be independent of dosage [16] and is unaffected or only slightly affected by food [17].
Systemic toxicity of fludarabine
The most frequent adverse events associated with fludarabine regimens are myelosuppression (neutropenia, thrombocytopenia and anemia), lymphocytopenia and infection (typically respiratory tract infections and fever) [18]. Other toxicities include gastrointestinal adverse effects, such as nausea, vomiting and elevation of liver enzymes.
Myelosuppression is the major dose-limiting adverse effect associated with fludarabine therapy in cancer patients. In large-scale randomized studies, the administration of 479 fludarabine treatment cycles to 96 patients with CLL resulted in the development of granulocytopenia, thrombocytopenia and anemia (WHO grade III/IV) during 19, 14 and 7% of treatment cycles, respectively, and affected 38, 15 and 18% of patients, respectively, during the first six treatment cycles [19].
Fludarabine’s dramatic depletion of lymphocytic cells is associated with an increase in opportunistic infections that requires close monitoring and management [20]. The most frequent infectious complications are respiratory tract infections and unexplained fever. Fatal outcomes have also been reported [21]. Many of these opportunistic infections occur in cases where there was concomitant corticosteroid use [22].
Neurotoxicity of fludarabine
Although myelosuppressive toxicity develops in almost half of the patients receiving fludarabine, regardless of dosage, a critical obstacle in the further use of fludarabine is its neurotoxicity. Higher doses of fludarabine for acute leukemia can be associated with severe neurotoxicity leading to encephalopathy, coma and even death in 18% of patients [23-26]. The development of neurologic toxicity is characterized by delayed onset (21-60 days after last treatment) and a progressive degenerative clinical course. The clinical symptoms consist of altered mental status, seizures, paraparesis, progressive encephalopathy and coma. The results of diagnostic studies, including CSF examination, electroencephalogram and CT scans of the CNS, are also varied and nonspecific. MRI studies have shown extensive diffuse loss of white matter [25].
Spriggs et al. reported that 11 patients with relapsed acute leukemia received 14 courses of fludarabine phosphate as a 5-day continuous infusion administered at doses of 40-100 mg/m2/day [24]. Three of these patients (27.3%) suffered neurotoxicity. Two of these three patients had a severe neurotoxicity syndrome characterized by blindness, encephalopathy and coma. Chun et al. reported that 13 out of 36 patients (36.1%) who received fludarabine at high doses (≥ 96 mg/m2/day for 5-7 days) developed neurotoxicity after receiving the drug [23]. Among these 13 patients, progressive deterioration of mental status or encephalopathy leading to a vegetative state developed in 11 patients. CT scan of the brain revealed no specific abnormalities except cortical atrophy in two patients. Follow-up MRI scans of these patients demonstrated an extensive diffuse loss of white matter. Analyses of CSF revealed an elevated protein level in five out of ten patients and ten out of ten negative cytologic examinations. The myelin-basic protein level was elevated in CSF from four out of ten patients examined. Visual-evoked potentials (VEP) were absent in three out of ten patients. The incidence and main manifestations of neurotoxicity with high-dose fludarabine treatment are listed in TABLE 1.
Table 1.
Neurotoxicity and ocular toxicity of high-dose fludarabine
| Study | Year | n | Dose/schedule | Patients with neurotoxicity (n) | Neurotoxicity | Patients withocular toxicity (n) | Ocular toxicity | Patients with vision recovery (n) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Hutton et al. | 1984 | 13 | 18-40 mg/m2/day over 30 min × 5 days | 8 | Somnolence during infusion, quick cleared (n = 8) | NS | NS | NS | [53] |
| Warrell et al. | 1986 | 25 | 50 mg/m2/d CIV × 5 day; 125 mg/m2/d CIV × 7 days |
5 | Mental status change (n = 2); quadriparesis (n = 1); seizures (n =1); coma (n = 4); paralysis (n = 4) |
5 | Amaurosis (n = 1); Blurred vision (n = 1); Cortical blindness (n = 5) |
1 | [25] |
| Chun et al. | 1986 | 36 | >96 mg/m2/day CIV × 5 days | 13 | Encephalopathy (n = 7); coma (n = 6); spastic paraparesis (n = 3) |
11 | Hallucination (n = 1); Bilateral papillitis (n = 1); blurred vision (n = 1); loss of vision (n = 8); cortical blindness (n = 2) |
1 | [23] |
| Spriggs et al. | 1986 | 11 | 40-100 mg/m2/day CIV × 5 days | 3 | Encephalopathy (n = 2); coma (n = 2); resting tremor (n = 1) | 2 | Blindness (n = 2) | 0 | [24] |
CIV: Continuous intravenous infusion; NS: Not stated.
As a result of the severe toxic and occasionally lethal side effects of fludarabine, interest in fludarabine as a treatment for AML waned. However, careful examination of the Phase I/II clinical trial data revealed that neurotoxicity appears to be dose related. In Chun’s report, 13 out of 36 patients (36.1%) who received fludarabine at high doses (≥ 96 mg/m2/day for 5-7 days per course) developed neurotoxicity, while only 1 out of 443 patients (0.2%) who received the drug at lower doses (less than or equal to 125 mg/m2 per course, equal to 25 mg/m2/day for 5 days), developed similar toxicity [23]. This one patient represents the first reported case of cortical blindness, encephalopathy and death resulting from treatment with low-dose fludarabine. However, this patient also had a CNS mycosis fungoides, which may have allowed greater fludarabine penetration into the brain, with resultant neurotoxic sequelae at a lower dose.
In the early 1990s, lower doses of fludarabine (30 mg/m2 per day for 5 days) were used successfully without neurotoxicity in the treatment of CLL, renewing interest in this agent [27-29]. Most large studies have reported no or few severe neurological side effects with standard-dose fludarabine therapy [30.31]. Investigators from a large European study reported the development of severe peripheral neuropathy (Grade III/IV) in two out of 479 fludarabine treatment cycles [19]. In 1994, Cheson et al. reviewed the literature for reports of adverse drug reactions from treatment with fludarabine [26]. In his review, 335 out of 2136 (16%) patients treated for a range of hematological malignancies with standard-dose fludarabine demonstrated neurotoxicity. The majority of cases were mild and reversible and the incidence was similar to that reported for cladribine [26]. Cases of adverse neurological events from treatment with low-dose fludarabine included both reversible neurotoxicity (seizures, loss of consciousness, blurred vision and leg weakness) and fatal neurotoxicity (multifocal leukoencephalopathy), and are listed in TABLE 2.
Table 2.
Neurotoxicity and ocular toxicity of low-dose fludarabine
| Study | No. of patients | Dose/schedule | Patients with neurotoxicity (n) | Neurotoxicity | Patients with ocular toxicity (n) | Ocular symptoms | Patients with vision recovery (n) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chun et al. (1986) | 443 | 18-22.5 mg/m2/day × 5 days, 3 course | 1 | Weakness, somnolence, disorientation, encephalopathy, coma (n = 1) | 1 | Hallucinations; complete blindness |
0 | [23] |
| Harvey et al. (1987) | 19 | 18.75-31.25 mg/m2/day × 5 days | 7 | Mild somnolence during infusion (n = 7); mild paresthesias (n = 1) |
NS | NS | NS | [54] |
| Balducci et al. (1987) | 36 | 25 mg/m2/day × 5 days | 2 | Somnolence (n = 2) | NS | NS | NS | [55] |
| Weiss et al. (1986) | 23 | 18-22mg/m2/day × 5 days | 11 | Mild-to-moderate somnolence or fatigue; transient paresthesias (n = 10), dementia, coma, death (n = 1) |
NS | NS | NS | [56] |
| Rainey et al. (1988) | 11 | 18 mg/m2/day × 5 days, with 25% dose escalation | 2 | Severe ataxia (n = 1); somnolence (n = 2); dizziness (n = 1) |
0 | 0 | 0 | [57] |
| Von Hoff et al. (1990) | 20 | 18 mg/m2/day × 5 days | 1 | Transient somnolence (n = 1) | NS | NS | NS | [44] |
| Kantarjian et al. (1991) | 17 | 30 mg/m2/day × 5 days | 1 | Mild sensory neuropathy (n = 1) | 0 | 0 | 0 | [58] |
| Hochster et al. (1992) | 62 | 18 mg/m2/day × 5 days | 32 | Mild (n = 17); moderate (n = 9); severe ( n = 6) |
2 | Visual changes | NS | [59] |
| Cohen et al. (1993) | NS | 18-25 mg/m2/day × 5 days, 6-8 cycles | 2 | Focal motor seizure (n = 1); loss of consciousness, improved rapidly in 6 weeks (n = 1); gait disturbance (n = 1) |
1 | Blurred vision (n=1)* | Reversible after 6 months | [60] |
| Bishop et al. (2007) | 1 | 25 mg/m2/day × 5 days, 2 cycles | 0 | Spinal cord syndrome Encephalomyelopathy | 1 | Visual changes | 0 | [UNPUBLISHED DATA] |
| Bishop et al. (2007) | 1 | 30 mg/m2/day × 4 days | 2 | Leukoenchaphalopathy (pre-existing but progressed postfludarabine) | 1 | Visual changes | Slight recovery | [UNPUBLISHED DATA] |
General anesthesia 1 day before the vision symptoms.
NS: Not stated.
Postmortem examination of the CNS in cases of fludarabine toxicity revealed various degrees of demyelination, either multifocal or diffuse, in the brain and spinal cord [32]. The most striking findings were within the cerebral white matter. These areas showed a diffuse, necrotizing leukoencephalopathy, characterized by vacuolization, myelin loss with numerous PAS-positive macrophages and axonal swelling with spheroid formation.
Visual deficits were the most common presenting symptom and eventually developed in most cases with neurotoxicity [23]. Examination of the brain at autopsy revealed significant necrosis within the occipital and parietal lobes, while the frontal and temporal lobes were less extensively involved. These observations suggest that the mechanism of toxicity may be related to impairment of oligodendroglial or axonal function, which is most apparent in those areas of the brain with the greatest metabolic activity.
Ocular toxicity of fludarabine
Specific ocular toxicities caused by fludarabine have been documented, although they are infrequent. Opportunistic infections have been reported in the eye. Reactivation of varicella zoster virus in the eye including the anterior segment (cornea and conjunctiva) and posterior segment (acute retinal necrosis syndrome, [ARNS]) has been reported. Chee et al. described two patients treated with fludarabine who developed progressive bilateral visual loss with anterior uveitis, vitritis, retinal vasculitis and peripheral retinal necrotic lesions [33]. One patient had received five courses of 25 mg/m3 for 3 days. At 2 weeks after treatment this patient developed progressive bilateral vision loss, was diagnosed with acute retinal necrosis, and then treated with high-dose intravenous acyclovir. Treatment prevented formation of new retinal lesions but did not improve visual acuity. The second patient received six courses of 25 mg/m3 for 5 days. Approximately 1 year after treatment he developed floaters and left-sided visual loss. The patient was treated with intravenous acyclovir, followed by oral famciclovir, with resolution of bilateral vitritis and return of visual acuity to baseline.
Among the 13 patients receiving high-dose fludarabine (≥96 mg/m2/day for 5-7 days per course) in Chun’s study, 11 developed ocular toxicity, eight experienced complete loss of vision, two demonstrated cortical blindness and one described blurred vision [23]. Ocular findings were varied and included visual changes, hallucinations, visual field deficits, optic neuritis, papillitis and cortical blindness. Visual abnormalities were sometimes the initial presenting symptom in some patients. Spriggs et al. described a 32-year-old male who had received fludarabine (100 mg/m2/day for 5 days), who initially complained of a slight decrease in visual acuity and photophobia 44 days after starting the medication [24]. The patient declined to no light perception vision within 3 days, followed by a generalized deterioration of mental status.
Ocular susceptibility to fludarabine toxicity is not limited to high-dose therapy. The patients surveyed by the Group C Protocol Mechanism of the National Cancer Institute represent the largest clinical investigation to date [34]. This trial enrolled more patients and had longer follow-up than any prior published trials and thereby provided valuable information on the toxicity profile of fludarabine [34]. In this study, 1 and 0.3% of patients developed Grade 3 and Grade 4 visual toxicity, respectively. The grading system was based on the common toxicity criteria of the National Cancer Institute. Grade 3 was defined as generalized symptomatic subtotal loss of vision, whereas Grade 4 was defined as blindness.
Recently, we have seen two patients who suffered total vision loss after receiving standard doses of fludarabine (25 mg/m2/day for 5 days). The first patient had stage 4 malignant melanoma and received two cycles of fludarabine as part of a conditioning regimen in preparation for adoptive cell therapy, with the first cycle including total body irradiation. The second patient had a diagnosis of systemic lupus erythematosus (SLE) and received fludarabine prestem cell transplantation. The first patient, with 20/25 vision in both eyes, noted visual aberrations including floaters and hallucinations, approximately 1 month after beginning her second course of fludarabine. She then experienced rapid decline of vision within 3 days, deteriorating to 20/640 in the right eye and 20/800 in the left eye. Electroretinography at the time of vision loss revealed a dramatic decrease in retinal bipolar cell function. Over the ensuing weeks prior to her death, her vision deteriorated to light perception in the right eye, and possibly no light perception in the left. Ocular autopsy disclosed extensive loss of retinal ganglion cells (FIGURE 1), strong immunoreactivity against glial fibrillary acidic protein (GFAP) and CD68+ cells in the areas of the optic nerve head and retinal inner layers of the posterior pole, including the macula, suggestive of gliosis and infiltration of microglia and macrophage. Protein kinase C (PKC-α), a marker of bipolar cells, was markedly decreased in the macular area. Our second similar case involved a 23-year-old African-American male who had received fludarabine prior to stem cell transplantation for SLE. Over the course of the patient’s illness, he experienced fluctuations in visual acuity, with deterioration to possible light perception in both eyes. Pathological examination revealed neuronal cellular atrophy, hydrocephalus and cerebral edema with inferior cerebellar herniation. As with our first patient, dramatic loss of ganglion cells (FIGURE 2), loss of bipolar cells and GFAP-positive gliosis in the ganglion cell layer of the macula were seen. In addition, many microglia cells were found in the optic nerve. Antiretinal antibodies from both patients’ sera collected prior to and after fludarabine treatment were negative. No other factors were identified that might have predisposed these two patients to experience such severe toxic reactions to fludarabine.
Figure 1. Photomicrograph of the retina discloses drastic reduction of ganglion cell layer from five to six cells to one cell layer in the macular area.
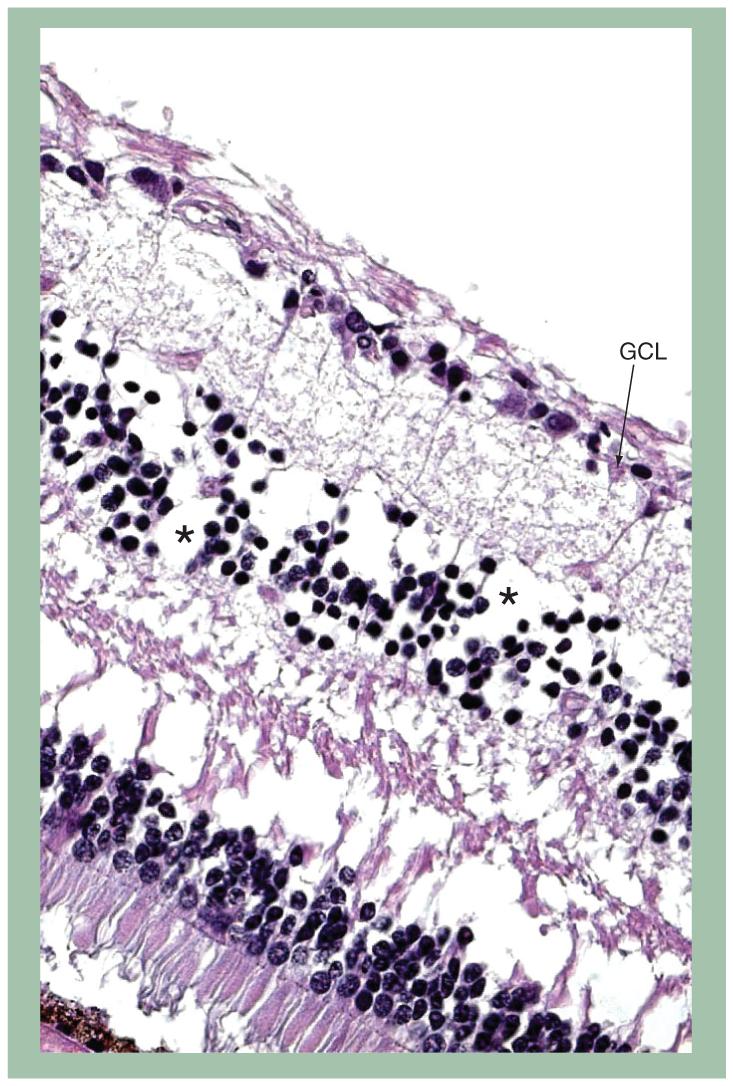
Loss of cells (in the inner nuclear layer) is also noted.
Hematoxylin and eosin stain, original magnification, ×100
*Bipolar cells.
GCL: Ganglion cell layer.
Figure 2. Photomicrograph of the retina illustrates similar changes to FIGURE 1 with severe decrease of ganglion cells and partial loss of bipolar cells in the macular area.
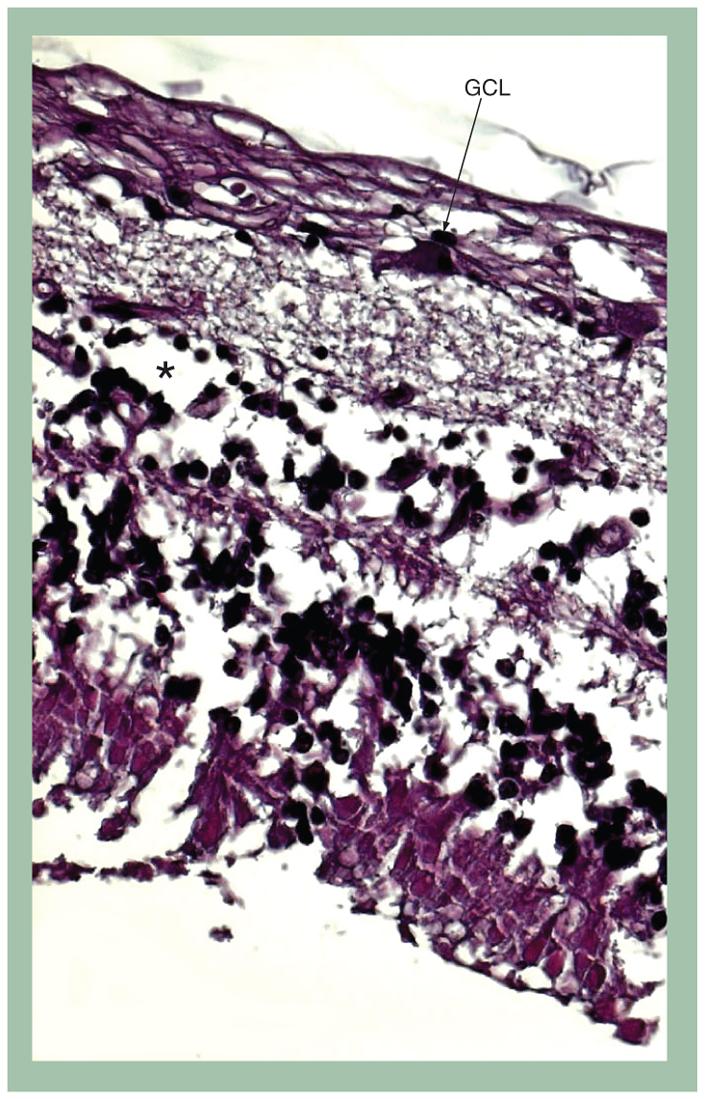
Hematoxylin and eosin stain, original magnification, ×100.
*Bipolar cells.
GCL: Ganglion cell layer.
In the previous report, postmortem examination of the optic nerves post-fludarabine toxicity revealed severe necrosis with myelin loss, numerous periodic acid schiff (PAS)-positive macrophages, and early reactive astrocytosis [24]. The necrosis was most severe within the occipital and parietal lobes. Stillman’s case of high-dose fludarabine neurotoxicity demonstrated multiple areas of leukoencephalopathy involving in particular the optic nerves, chiasm and tract [35]. Postmortem examination of our two patients also revealed extensive optic nerve atrophy.
Neurotoxicity from fludarabine appears to be largely irreversible. Visual recovery, however, has been seen in some cases with immediate cessation of fludarabine administration at the first signs of neurotoxicity. For example, in Warrell’s report one of the five patients who had developed total blindness and quadriparesis gradually regained both vision and strength [25]. The patient’s only permanent neurologic deficit was an asymptomatic delay in visually evoked response. On the other hand, in Chun’s study only one of the 13 patients who had experienced visual loss recovered vision [23].
The increasing clinical use of fludarabine necessitates a heightened awareness of the neurological vulnerability of some patients to low doses of fludarabine. The potential life-and sight-threatening consequences can be significant. The mechanisms of neurotoxicity may be multifactorial and at present cannot be predicted. Continued caution in the use of this antineoplastic agent is appropriate.
Combination therapy with fludarabine
There is a substantial body of evidence supporting the hypothesis that fludarabine potentiates the activity of other antitumor agents, such as cisplatin, cytarabine, mitoxantrone, and cyclophosphamide [4,36].
Combination chemotherapy with other antimetabolites
The efficacy of a combination regimen of cytarabine, currently the most widely used single agent in the treatment of AML, plus low-dose fludarabine has been investigated [37.38]. There is a direct correlation between the ability of leukemic blasts to form and retain Ara-CTP, and the clinical response of patients with AML to high-dose cytarabine. The rationale for this combination was based on the discovery that fludarabine is able to modulate the metabolism of cytarabine in vitro, thereby increasing the accumulation of Ara-CTP [37,38]. Studies were therefore designed with fludarabine infusions (30 mg/m2) preceding cytarabine infusions, in order to enhance Ara-CTP accumulation. The regimen appears to be clinically effective. Assessment of its potential neurotoxicity is important because fludarabine, as noted previously, and cytarabine may independently produce neurological damage [39].
Kornblau et al. reported an overall incidence of neurotoxicity of 3.6% in eight out of 219 patients who received a combination of fludarabine and cytarabine [40]. In total, five patients developed peripheral neuropathy but there was no association with age, creatinine, dose of cytarabine or number of courses. Two patients developed severe progressive cerebral dysfunction that was ultimately fatal. The toxicity was similar to that seen with high-dose fludarabine therapy. Both of these patients were older than 60 years and had a serum creatine greater than or equal to 2.0 mg/dl. Since fludarabine is partially excreted by the kidneys, toxicity in these two patients was probably due to effectively receiving a high dose of fludarabine. Neither toxicity was observed in the 481 CLL patients treated with fludarabine alone at the same dose and on the same schedule, suggesting that combination with cytarabine is associated with the development of the peripheral neuropathy. In the eight patients with onset of neurological symptoms, only one experienced visual loss. Funduscopy and ophthalmic pathology of this patient were not described [40]. The incidence of neurotoxicity with the combination of fludarabine and cytarabine is still low in comparison with high-dose cytarabine therapy (3 g/m2 over 2 h).
In Chun’s report, five out of 13 patients with CNS toxicity had received prior high-dose cytarabine, and four had received prior intrathecal chemotherapy with either methotrexate, cytarabine or both [23]. In fact, two of the 13 patients had a residual neurologic deficit from prior therapy at the time of entry to the fludarabine trial. Among the 23 patients without neurotoxicity receiving similar high-dose fludarabine, three patients had received prior high-dose cytarabine and eight had received prior intrathecal chemotherapy [23]. Based on these findings, the authors suggest that prior high-dose cytarabine appears not to be a predisposing factor for the development of CNS toxicity after fludarabine [23].
Fludarabine combined with alkylating agents
Fludarabine combined with cyclophosphamide
Coadministration of fludarabine and cyclophosphamide is the most fully investigated fludarabine combination. It has been examined in several trials, including trials with additional drugs such as filgrastim and mitoxantrone. In a Phase III trial of 362 patients with treatment-naive CLL, the overall response (OR), complete response (CR), progression-free survival time and treatment-free survival time were significantly higher in the combination group than in the fludarabine or cyclophosphamide monotherapy groups. The most common adverse event associated with the combination of fludarabine with cyclophosphamide was myelosuppression. Myelotoxicity, in particular leukocytopenia and thrombocytopenia, was significantly more frequent in the combination regimen. In spite of the higher rate of severe leukocytopenia, the incidence of severe infections was similar in both treatment groups [41]. A possible explanation is that the fludarabine and cyclophosphamide combination dosing was more frequently reduced or delayed as compared with fludarabine monotherapy. Gastrointestinal side effects such as nausea, vomiting, mucositis and gastritis were more common in the combined therapy group. No neurotoxicity or ocular toxicity has been associated with this combination, with fludarabine dosed at 96 mg/m2/day for 5-7 days per course.
Recently, a large-scale randomized controlled trial studying 777 CLL patients reported a lower incidence of hemolytic anemia when using fludarabine plus cyclophosphamide (5%) than with fludarabine (11%) alone [42]. A meta-analysis of these data combined with two published Phase III trials revealed a consistent benefit from the fludarabine/cyclophosphamide regimen combination with respect to progression-free survival. In addition, responders in the combination group reported a higher quality of life. Ocular toxicity was not reported in this combination, with fludarabine dosed at 96 mg/m2/day for 5-7 days per course.
Fludarabine combined with chlorambucil
A subsequent retrospective analysis revealed a significantly higher incidence of major infections among patients who received combination therapy with fludarabine and chlorambucil, requiring hospitalization or treatment with parenteral antibiotics [43]. Incidences of major infections were 29, 17, and 45% in the fludarabine, chlorambucil and fludarabine plus chlorambucil groups, respectively [44]. No ocular toxicity was mentioned.
Fludarabine combined with immunomodulating agents
Fludarabine is now used in combination with thalidomide, a first-generation immunomodulating agent that downregulates TNF-α and VEGF, to treat patients with CLL. A recent Phase I clinical trial of thalidomide in combination with fludarabine showed a high (100%) OR rate in treatment-naive patients with CLL, with 55% of patients achieving complete remission [45]. In this study, the most common toxicities noted were fatigue, constipation and peripheral sensory neuropathy. No severe neurotoxicity was noted. In a separate investigation involving daily treatments with thalidomide, oral fludarabine, and oral cyclophosphamide, one out of five patients with a prior history of Guillain-Barré syndrome, had to stop treatment after developing a sensory motor neuropathy [46]. Ocular toxicity was not reported in this study.
In conclusion, the combination of fludarabine with other chemotherapeutics does not appear to significantly change the adverse event profiles previously described. Most of the investigations of myelotoxicity and neurotoxicity following fludarabine combination therapy suggest that the incidence of these complications may be higher than previously reported for fludarabine monotherapy [46,47]. However, owing to differences among the populations of patients, combinations of chemotherapy agents and absence of specific toxicity data, it is unclear how much greater the complication rate of combination therapy might actually be.
Expert commentary & five-year view
Progressive demyelination of the CNS is the suggested pathologic process occurring after fludarabine phosphate treatment. Although ocular pathology confirms loss of ganglion cells and damage of bipolar cells, which could be due to direct neuronal toxicity from fludarabine and/or retrograde neuronal atrophy, the precise mechanism responsible for the injury to particular neuronal cells is unknown. Understanding the pharmacokinetic behavior and precise action of fludarabine in the CNS may further help the understanding of the pathophysiology. Further investigations, including in vitro and in vivo studies in animals will be necessary for a better understanding of fludarabine neurotoxicity.
A major clinical challenge in cancer treatment currently is identifying and managing individual variability to the drug regimen selected. Although fludarabine is highly active in lymphoproliferative disorders, a significant portion of patients are resistant, whereas others are susceptible to the toxicity of this agent. Some studies suggested that heterogenous responses to identical fludarabine treatment regimens may be explained, at least in part, by individual variability in the expression of certain gene products, such as the Concentrative Nucleoside Transporters (CNTs) [48,49]. CNTs are located on the apical membrane of the intestine and liver epithelia. This suggests that they may play an important role in the absorption and deposition of nucleosides. CNT2, and CNT3 mRNAs were also expressed in rat retinal capillary endothelial cells, which were used as an in vitro model of the inner blood-retinal barrier [50]. As naturally occurring nucleosides and most synthetic nucleoside analogs are hydrophilic and require nucleoside transporters to traverse biological membranes, nucleoside transporters are critical determinants of cellular and whole-body homeostasis of nucleosides and are important players in the tissue-specific disposition and pharmacokinetics of nucleoside analog drugs. It is reported that CNT3 is primarily responsible for the transport of several antileukemic drugs, including fludarabine and cladribine [48.49]. Badagnani and associates analyzed the genetic variants in the human CNT3; SLC29A3 [51]. Analysis of expression levels of nucleoside transporters in leukemic cells suggests that CNT3 has the highest interindividual variability. In addition, Gray et al. recently reported that several genetic variants of CNT1 exhibited altered interaction with gemcitabine, suggesting that common CNT1 variants may contribute to variation in systemic and intracellular levels of pyrimidine nucleoside analog drugs [52]. These genes may play an important role in mediating the cellular entry of a broad array of synthetic anticancer nucleoside analog drugs such as fludarabine. Further research on the pharmacogenetics of the drug may lead to a clearer explanation of the individual variability in toxicity seen during treatment.
With increased clinical use of fludarabine, its known toxicities of myelosuppression and immunosuppression have become more apparent. Myelosuppression can be managed with the use of growth factors, and infectious complications can be mitigated with adequate prophylactic antibiotics. However, there is no known prophylaxis or treatment for neurotoxicity to date, in particular to ocular toxicity. Increased awareness of potentially serious side effects and close observation of patients using this drug are recommended in the use of both high and low doses of fludarabine.
Key issues.
Ocular toxicities induced by fludarabine are infrequent but may be rapidly sight-threatening and largely irreversible.
Susceptibility to fludarabine ocular toxicity is not limited to high-dose therapy.
Pathology of eyes with fludarabine toxicity demonstrates atrophy of the optic nerve and inner retina, infiltration of microglia/macrophages and marked gliosis.
The precise mechanism responsible for fludarabine toxicity remains an enigma.
The incidence of neurotoxicity from the combination of fludarabine with other drugs may be higher than that from fludarabine alone.
Increased awareness of potentially serious side effects and close observation of patients using this drug is recommended in the use of both high and low doses of fludarabine.
A better understanding of interindividual variability in the effects of fludarabine might be attained through the elucidation of fludarabine metabolism in the CNS, including the pharmacokinetic profile of fludarabine and the pharmacogenetic factors influencing fludarabine activity and/or elimination.
Acknowledgments
Financial & competing interests disclosure
This article was funded by the NEI Intramural Research Program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Contributor Information
Xiaoyan Ding, Section of Immunopathology, Laboratory of Immunology, National Eye Institute, National Institutes of Health, Bethesda, MD, USA; And, Zhongshan Ophthalmic Center, Sun Yet-sen University, Guangzhou, China.
Alexandra A Herzlich, Section of Immunopathology, Laboratory of Immunology, National Eye Institute, National Institutes of Health, Bethesda, MD, USA.
Rachel Bishop, Clinical Branch, National Eye Institute, National Institutes of Health, Bethesda, MD, USA.
Jingsheng Tuo, Section of Immunopathology, Laboratory of Immunology, National Eye Institute, National Institutes of Health, Bethesda, MD, USA.
Chi-Chao Chan, 10 Center Drive, Building 10, Room 10N103, NIH/NEI Bethesda, MD 20892-1857 USA.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Blau IW, Schmidt-Hieber M, Leschinger N, et al. Engraftment kinetics and hematopoietic chimerism after reduced-intensity conditioning with fludarabine and treosulfan before allogeneic stem cell transplantation. Ann. Hematol. 2007;86(8):583–589. doi: 10.1007/s00277-007-0294-6. [DOI] [PubMed] [Google Scholar]
- 2.Karp JE, Giles FJ, Gojo I, et al. A Phase I study of the novel ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine® in combination with the nucleoside analog fludarabine for patients with refractory acute leukemias and aggressive myeloproliferative disorders. Leuk. Res. 2007;32(1):71–77. doi: 10.1016/j.leukres.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery JA, Hewson K. Nucleosides of 2-fluoroadenine. J. Med.Chem. 1969;12(3):498–504. doi: 10.1021/jm00303a605. [DOI] [PubMed] [Google Scholar]
- 4.Adkins JC, Peters DH, Markham A. Fludarabine. An update of its pharmacology and use in the treatment of haematological malignancies. Drugs. 1997;53(6):1005–1037. doi: 10.2165/00003495-199753060-00007. [DOI] [PubMed] [Google Scholar]
- 5.Plosker GL, Figgitt DP. Oral fludarabine. Drugs. 2003;63(21):2317–2323. doi: 10.2165/00003495-200363210-00004. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin. Pharm. 2002;41(2):93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- 7.Tseng WC, Derse D, Cheng YC, Brockman RW, Bennett LL., Jr. In vitro biological activity of 9-β-d-arabinofuranosyl-2-fluoroadenine and the biochemical actions of its triphosphate on DNA polymerases and ribonucleotide reductase from HeLa cells. Mol. Pharmacol. 1982;21(2):474–477. [PubMed] [Google Scholar]
- 8.White EL, Shaddix SC, Brockman RW, Bennett LL., Jr. Comparison of the actions of 9-β-d-arabinofuranosyl-2-fluoroadenine and 9-β-d-arabinofuranosyladenine on target enzymes from mouse tumor cells. Cancer Res. 1982;42(6):2260–2264. [PubMed] [Google Scholar]
- 9.Avramis VI, Plunkett W. Metabolism of 9-β-d-arabinosyl-2-fluoroadenine-5′-phosphate by mice bearing P388 leukemia. Cancer Drug Deliv. 1983;1(1):1–10. doi: 10.1089/cdd.1983.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Noker PE, Duncan GF, El Dareer SM, Hill DL. Disposition of 9-β-d-arabinofuranosyl-2-fluoroadenine 5′-phosphate in mice and dogs. Cancer Treat. Rep. 1983;67(5):445–456. [PubMed] [Google Scholar]
- 11.Keating MJ, O’Brien S, McLaughlin P, et al. Clinical experience with fludarabine in hemato-oncology. Hematol. Cell Ther. 1996;38(Suppl 2):S83–S91. [PubMed] [Google Scholar]
- 12.Malspeis L, Grever MR, Staubus AE, Young D. Pharmacokinetics of 2-F-ara-A (9-β-d-arabinofuranosyl-2-fluoroadenine) in cancer patients during the Phase I clinical investigation of fludarabine phosphate. Semin. Oncol. 1990;17(5 Suppl 8):18–32. [PubMed] [Google Scholar]
- • Describes the pharmacokinetics characteristics of fludarabine in humans during the Phase I clinical investigation.
- 13.Hersh MR, Kuhn JG, Phillips JL, et al. Pharmacokinetic study of fludarabine phosphate (NSC 312887) Cancer Chemother. Pharmacol. 1986;17(3):277–280. doi: 10.1007/BF00256699. [DOI] [PubMed] [Google Scholar]
- 14.Lichtman SM, Etcubanas E, Budman DR, et al. The pharmacokinetics and pharmacodynamics of fludarabine phosphate in patients with renal impairment: a prospective dose adjustment study. Cancer Invest. 2002;20(78):904–913. doi: 10.1081/cnv-120005903. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi V, Kemena A, Keating MJ, Plunkett W. Cellular pharmacology of fludarabine triphosphate in chronic lymphocytic leukemia cells during fludarabine therapy. Leuk. Lymphoma. 1993;10(12):49–56. doi: 10.3109/10428199309147356. [DOI] [PubMed] [Google Scholar]
- 16.Foran JM, Oscier D, Orchard J, et al. Pharmacokinetic study of single doses of oral fludarabine phosphate in patients with ‘low-grade’ non-Hodgkin’s lymphoma and B-cell chronic lymphocytic leukemia. J. Clin. Oncol. 1999;17(5):1574–1579. doi: 10.1200/JCO.1999.17.5.1574. [DOI] [PubMed] [Google Scholar]
- 17.Oscier D, Orchard JA, Culligan D, et al. The bioavailability of oral fludarabine phosphate is unaffected by food. Hematol. J. 2001;2(5):316–321. doi: 10.1038/sj.thj.6200113. [DOI] [PubMed] [Google Scholar]
- 18.Yildiz O, Ozguroglu M, Yanmaz MT, et al. Paraneoplastic pemphigus associated with fludarabine use. Medical oncology. 2007;24(1):115–118. doi: 10.1007/BF02685912. [DOI] [PubMed] [Google Scholar]
- 19.Johnson S, Smith AG, Loffler H, et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet. 1996;347(9013):1432–1438. doi: 10.1016/s0140-6736(96)91681-5. [DOI] [PubMed] [Google Scholar]
- 20.Montillo M, Ricci F, Tedeschi A. Role of fludarabine in hematological malignancies. Expert Rev. Anticancer. Ther. 2006;6(9):1141–1161. doi: 10.1586/14737140.6.9.1141. [DOI] [PubMed] [Google Scholar]
- 21.Anaissie EJ, Kontoyiannis DP, O’Brien S, et al. Infections in patients with chronic lymphocytic leukemia treated with fludarabine. Ann. Intern. Med. 1998;129(7):559–566. doi: 10.7326/0003-4819-129-7-199810010-00010. [DOI] [PubMed] [Google Scholar]
- 22.Girmenia C, Mauro FR, Rahimi S. Late listeriosis after fludarabine plus prednisone treatment. Br. J. Haematol. 1994;87(2):407–408. doi: 10.1111/j.1365-2141.1994.tb04932.x. [DOI] [PubMed] [Google Scholar]
- 23.Chun HG, Leyland-Jones BR, Caryk SM, Hoth DF. Central nervous system toxicity of fludarabine phosphate. Cancer Treat. Rep. 1986;70(10):1225–1228. [PubMed] [Google Scholar]
- •• Study suggests the development of neurotoxicity of fludarabine is highly dose-dependent.
- 24.Spriggs DR, Stopa E, Mayer RJ, Schoene W, Kufe DW. Fludarabine phosphate (NSC 312878) infusions for the treatment of acute leukemia: Phase I and neuropathological study. Cancer Res. 1986;46(11):5953–5958. [PubMed] [Google Scholar]
- 25.Warrell RP, Jr, Berman E. Phase I and II study of fludarabine phosphate in leukemia: therapeutic efficacy with delayed central nervous system toxicity. J. Clin. Oncol. 1986;4(1):74–79. doi: 10.1200/JCO.1986.4.1.74. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Vena DA, Foss FM, Sorensen JM. Neurotoxicity of purine analogs: a review. J. Clin. Oncol. 1994;12(10):2216–2228. doi: 10.1200/JCO.1994.12.10.2216. [DOI] [PubMed] [Google Scholar]
- • Review on the adverse drug reaction of fludarabine that found that neurotoxicity was reported in 16% of patients with a range of hematological malignancies.
- 27.Grever M, Leiby J, Kraut E, et al. A comprehensive Phase I and II clinical investigation of fludarabine phosphate. Semin. Oncol. 1990;17(5 Suppl 8):39–48. [PubMed] [Google Scholar]
- 28.Von Hoff DD. Phase I clinical trials with fludarabine phosphate. Semin. Oncol. 1990;17(5 Suppl 8):33–38. [PubMed] [Google Scholar]
- 29.Grever MR, Kopecky KJ, Coltman CA, et al. Fludarabine monophosphate: a potentially useful agent in chronic lymphocytic leukemia. Nouv. Rev. Fr. Hematol. 1988;30(56):457–459. [PubMed] [Google Scholar]
- 30.Montserrat E, Lopez-Lorenzo JL, Manso F, et al. Fludarabine in resistant or relapsing B-cell chronic lymphocytic leukemia: the Spanish Group experience. Leuk. Lymphoma. 1996;21(56):467–472. doi: 10.3109/10428199609093445. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien ME, Matutes E, Cunningham D, et al. Fludarabine in lymphoproliferative disorders: the Royal Marsden Hospital experience. Leuk. Lymphoma. 1994;14(Suppl 2):17–23. doi: 10.3109/10428199409052691. [DOI] [PubMed] [Google Scholar]
- 32.Morgan AE. Chemotherapy-induced neurotoxicity. Cancer Control. 1995;2(3):235–242. [PubMed] [Google Scholar]
- 33.Chee YL, Culligan DJ, Olson JA, et al. Sightthreatening varicella zoster virus infection after fludarabine treatment. Br. J. Haematol. 2000;110(4):874–875. doi: 10.1046/j.1365-2141.2000.02206.x. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen JM, Vena DA, Fallavollita A, Chun HG, Cheson BD. Treatment of refractory chronic lymphocytic leukemia with fludarabine phosphate via the group C protocol mechanism of the National Cancer Institute: five-year follow-up report. J. Clin. Oncol. 1997;15(2):458–465. doi: 10.1200/JCO.1997.15.2.458. [DOI] [PubMed] [Google Scholar]
- •• Largest clinical investigation on fludarabine, which provides valuable information on the toxicity profile of the regimen.
- 35.Stillman M, Navia B, Mast J. Delayed neurotoxicity associated with fludarabine. Neurology. 1985;35(Suppl 1):291. [Google Scholar]
- 36.Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br. J. Haematol. 2001;114(4):800–809. doi: 10.1046/j.1365-2141.2001.03014.x. [DOI] [PubMed] [Google Scholar]
- 37.Seymour JF, Huang P, Plunkett W, Gandhi V. Influence of fludarabine on pharmacokinetics and pharmacodynamics of cytarabine: implications for a continuous infusion schedule. Clin. Cancer Res. 1996;2(4):653–658. [PubMed] [Google Scholar]
- 38.Santini V, D’Ippolito G, Bernabei PA, et al. Effects of fludarabine and gemcitabine on human acute myeloid leukemia cell line HL 60: direct comparison of cytotoxicity and cellular Ara-C uptake enhancement. Leuk. Res. 1996;20(1):37–45. doi: 10.1016/0145-2126(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 39.Gandhi V, Estey E, Keating MJ, Plunkett W. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J. Clin. Oncol. 1993;11(1):116–124. doi: 10.1200/JCO.1993.11.1.116. [DOI] [PubMed] [Google Scholar]
- 40.Kornblau SM, Cortes-Franco J, Estey E. Neurotoxicity associated with fludarabine and cytosine arabinoside chemotherapy for acute leukemia and myelodysplasia. Leukemia. 1993;7(3):378–383. [PubMed] [Google Scholar]
- 41.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J. Clin. Oncol. 2007;25(7):793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 42.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370(9583):230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 43.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2000;343(24):1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 44.Von Hoff DD, Green S, Surwit EA, Hannigan EV, Alberts DS. Phase II study of fludarabine phosphate (NSC 312887) in patients with advanced cervical cancer. A Southwest Oncology Group study. Am. J. Clin. Oncol. 1990;13(5):433–435. doi: 10.1097/00000421-199010000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Chanan-Khan A, Miller KC, Takeshita K, et al. Results of a phase 1 clinical trial of thalidomide in combination with fludarabine as initial therapy for patients with treatment-requiring chronic lymphocytic leukemia (CLL) Blood. 2005;106(10):3348–3352. doi: 10.1182/blood-2005-02-0669. [DOI] [PubMed] [Google Scholar]
- 46.Giannopoulos K, Dmoszynska A, Rolinski J. Low-dose thalidomide in combination with oral fludarabine and cyclophosphamide is ineffective in heavily pretreated patients with chronic lymphocytic leukemia. Leuk. Res. 2007;31(3):411–412. doi: 10.1016/j.leukres.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Tam CS, Seymour JF, Prince HM, et al. Treatment-related myelodysplasia following fludarabine combination chemotherapy. Haematologica. 2006;91(11):1546–1550. [PubMed] [Google Scholar]
- 48.Ritzel MW, Ng AM, Yao SY, et al. Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib) J. Biol. Chem. 2001;276(4):2914–2927. doi: 10.1074/jbc.M007746200. [DOI] [PubMed] [Google Scholar]
- 49.Mangravite LM, Badagnani I, Giacomini KM. Nucleoside transporters in the disposition and targeting of nucleoside analogs in the kidney. Eur. J. Pharm. 2003;479(13):269–281. doi: 10.1016/j.ejphar.2003.08.076. [DOI] [PubMed] [Google Scholar]
- 50.Nagase K, Tomi M, Tachikawa M, Hosoya K. Functional and molecular characterization of adenosine transport at the rat inner blood-retinal barrier. Biochim. Biophys. Acta. 2006;1758(1):13–19. doi: 10.1016/j.bbamem.2006.01.011. [DOI] [PubMed] [Google Scholar]
- • Study shows that CNT2 and CNT3 mRNAs were expressed in the inner blood-retinal barrier.
- 51.Badagnani I, Chan W, Castro RA, et al. Functional analysis of genetic variants in the human concentrative nucleoside transporter 3 (CNT3; SLC28A3) Pharmacogenomics J. 2005;5(3):157–165. doi: 10.1038/sj.tpj.6500303. [DOI] [PubMed] [Google Scholar]
- 52.Gray JH, Mangravite LM, Owen RP, et al. Functional and genetic diversity in the concentrative nucleoside transporter, CNT1, in human populations. Mol. Pharmacol. 2004;65(3):512–519. doi: 10.1124/mol.65.3.512. [DOI] [PubMed] [Google Scholar]
- 53.Hutton JJ, Von Hoff DD, Kuhn J, et al. Phase I clinical investigation of 9-β-d-arabinofuranosyl-2-fluoroadenine 5′-monophosphate (NSC 312887), a new purine antimetabolite. Cancer Res. 1984;44(9):4183–4186. [PubMed] [Google Scholar]
- 54.Harvey WH, Fleming TR, Von Hoff DD, Katterhagen JG, Coltman CA., Jr. Phase II study of fludarabine phosphate in previously untreated patients with colorectal carcinoma: a Southwest Oncology Group Study. Cancer Treat. Rep. 1987;71(12):1319–1320. [PubMed] [Google Scholar]
- 55.Balducci L, Blumenstein B, Von Hoff DD, et al. Evaluation of fludarabine phosphate in renal cell carcinoma: a Southwest Oncology Group Study. Cancer Treat. Rep. 1987;71(5):543–544. [PubMed] [Google Scholar]
- 56.Weiss GR, Crowley J, Von Hoff DD, et al. Phase II study of fludarabine phosphate for the treatment of advanced non-small cell carcinoma of the lung: a Southwest Oncology Group Study. Cancer Treat. Rep. 1986;70(9):1123–1124. [PubMed] [Google Scholar]
- 57.Rainey JM, Hill JB, Crowley J. Evaluation of fludarabine phosphate in small cell carcinoma. A Southwest Oncology Group Study. Invest. New Drugs. 1988;6(1):45–46. doi: 10.1007/BF00170779. [DOI] [PubMed] [Google Scholar]
- 58.Kantarjian HM, Childs C, O’Brien S, et al. Efficacy of fludarabine, a new adenine nucleoside analogue, in patients with prolymphocytic leukemia and the prolymphocytoid variant of chronic lymphocytic leukemia. Am. J. Med. 1991;90(2):223–228. [PubMed] [Google Scholar]
- 59.Hochster HS, Kim KM, Green MD, et al. Activity of fludarabine in previously treated non-Hodgkin’s low-grade lymphoma: results of an Eastern Cooperative Oncology Group study. J. Clin. Oncol. 1992;10(1):28–32. doi: 10.1200/JCO.1992.10.1.28. [DOI] [PubMed] [Google Scholar]
- 60.Cohen RB, Abdallah JM, Gray JR, Foss F. Reversible neurologic toxicity in patients treated with standard-dose fludarabine phosphate for mycosis fungoides and chronic lymphocytic leukemia. Ann. Intern. Med. 1993;118(2):114–116. doi: 10.7326/0003-4819-118-2-199301150-00007. [DOI] [PubMed] [Google Scholar]


