Abstract
A variety of anatomical features suggest that functional activity in the nervous system can influence the process of myelination, yet direct evidence of this is lacking. Research by Zalc and colleagues shows that myelination of optic nerve is inhibited by a neurotoxin that blocks action potential activity and is stimulated by a toxin that increases impulse activity, suggesting that impulse activity is necessary for initiating myelination during development of the optic nerve. Research by Fields and colleagues, using electrical stimulation of axons, shows that low frequency impulse activity inhibits myelination of dorsal root ganglion neurons, but high frequency impulse activity has no effect. This results from reduced expression of a cell adhesion molecule on the stimulated axons that is critical for inducing myelination. Together these studies support the conclusion that impulse activity can influence the process of myelination, probably through more than one molecular mechanism operating during discrete steps in the myelination process.
Keywords: Myelination, Activity-dependent, Action potential, Oligodendrocyte, Schwann cell, Dorsal root ganglion (DRG), Optic nerve, Cell adhesion molecule, L1, tetrodotoxin (TTX)
Emerging only after the evolution of early vertebrates, myelin dramatically elevated nervous function to unparalleled levels of information processing capacity and speed (1). In humans, the functional importance of myelin is seen in the growing cognitive and physical capabilities of young children (2, 3) and in the clinical dysfunction that accompanies injury to the myelin sheath in adults (4, 5). Myelin is an exquisite structure when seen at the ultrastructural level, and its structure has functional relevance. Correlations between the anatomy of myelin and physiological properties of axons suggest possible activity-dependent regulation of myelination.
Anatomical Evidence of Activity-Dependent Regulation of Myelination
It is clear that the axon participates in specifying features of myelination, including the distance between nodes of Ranvier and the thickness of the myelin sheath. Only axons become myelinated, never dendrites or cell bodies of neurons or non-neuronal cells (6). Small-diameter axons are not myelinated in peripheral nerve trunks (7), and ultrastructural analysis shows that premyelinated axons undergo membrane remodeling into foci with nodal properities before myelin is compacted (8–11).
The thickness of the myelin sheath and the internodal distance govern the conduction velocity of myelinated fibers (7). Rushton (7) noted that, along peripheral nerve trunks, myelin thickness and internode distance are adjusted so as to maximize conduction velocity and thereby optimize function. Interestingly, many cases are now known in which myelin is structured not to maximize the speed of conduction of action potentials but to ensure simultaneous arrival of impulses from multiple points that are separated by large differences in distance (12). The olivocerebellar projections carrying information to Purkinje cells in the cerebellum (13), the electromotor axons of certain electric fish (14–15), and the axons of retinal ganglion cells located at different eccentricities within the retina (16) show differences in conduction times that are adjusted by the structure of myelin, to provide simultaneous arrival of impulses within millisecond precision. Other evidence that the functional requirements of the axon can specify myelinating responses of glial cells is provided by observations that the internodal distance is reduced in specific regions of axons. This is often seen close to axon branch points where impedance matching is required for effective impulse propagation (7, 17). Also, along some axons, regions devoid of myelin are intercalated between normally myelinated regions at sites where they are needed to produce specific patterns of extracellular current (15). These observations all point to the conclusion that relationships between axons and myelinating glia are in some way regulated to meet the precise functional requirements of individual axons, and that an activity-dependent, functional feedback may contribute to the control of myelination.
The processes that maintain this structural/functional interrelationship between myelinating glia and axons have intrigued neuroscientists for some time. How do glia determine which axons should become myelinated and which should not? How is the distance between successive nodes of Ranvier established? What initiates the process of myelination at the appropriate point in development? (Typically myelination begins after the axon has reached its appropriate target, formed synapses, and begun firing action potentials, even though the glial cells have been closely associated with axons from much earlier in development.) How is the thickness of myelin so closely matched to the caliber of the axon? What maintains the relationship between myelin thickness and the caliber of the axon as the axon’s dimensions change throughout the life of an organism? There are probably many factors, both intrinsic and extrinsic, controlling myelination. Recognizing that the conduction properties of axons are a key variable, the possibility that impulse activity might influence the myelinating activity of oligodendrocytes or Schwann cells seems an attractive hypothesis.
Controversy on the Role of Impulse Activity on Myelination
Results of experiments on the role of impulse activity in regulating myelination are controversial and open to alternative interpretations. Early studies were interpreted as suggesting that increased impulse activity might promote the formation of myelin in the optic nerve. Mice reared in the dark developed fewer myelinated axons in the optic nerve compared with normally reared mice (18). Myelination has been noted to be highly decreased in the optic nerve of the naturally blind cape mole rat (19), whereas premature eye opening increased the level of myelin protein expression in the optic nerve of rabbit (20).
In contrast, other studies have reported that intraocular injections of the sodium channel blocker tetrodotoxin (TTX), which blocks action potential activity, had no effect on the number of myelinated fibers or the time of myelination onset in optic nerves of rat (21–23). In experiments on goldfish, action potential blockade by intraocular injection of TTX during optic nerve regeneration had no affect on myelination of regenerated fibers compared with control subjects (24). Dark-rearing of kittens (25) and rats (26) have been reported to have no affect on the initiation of myelination of the visual pathway during early postnatal development. These results in vivo are consistent with results of experiments on mouse spinal cord explants in vitro, which undergo normal oligodendrocyte development and myelination in the presence of TTX (27).
In cell culture, axonal signals are required at all stages of Schwann cell development into myelin-forming cells (28–32), but myelin synthesis by oligodendrocytes seems to be less dependent on axon contact. All the stages leading an oligodendrocyte progenitor to survive, proliferate, and differentiate into a postmitotic oligodendrocyte have been observed, in vitro, in neuron-free cultures (for review, see 33). Moreover, highly purified mature oligodendrocytes, when maintained in culture in the total absence of neurons, can extend, at the tip of their processes, rafts of membranes, which can even wrap around themselves to form myelin-like figures, or adhere to carbon fibers. Even under these circumstances, however, axons are needed for compaction of the myelin sheath. In neuron-free cultures, the pseudo-myelin structures present at the tip of oligodendroglial processes (flat layers of membrane), or the wending of these processes around carbon fibers, never display the characteristic, tight compaction of normal myelin surrounding an axon (16, 34). More recently, the timing of oligodendrocyte differentiation has been shown to be controlled by neurons through down-regulation of Jagged1 along axons (35). This finding corroborates the observation that highly purified mature oligodendrocytes up-regulate the transcription rate of the major myelin genes upon addition to cultures of neurons (36).
Studies of Electrical Activity in Myelination of Optic Nerve
To determine whether the onset of myelination was the consequence only of oligodendrocyte maturation, or was dependent on an axonal signal, one of the authors (BZ) and colleagues investigated the influence of axonal electrical activity on myelinogenesis (37). Neurons and oligodendrocyte progenitors dissociated from embryonic mouse brain hemispheres at day 15 of gestation were maintained in co-culture. Under these conditions, the first myelinated axons were seen after 13 to 15 days in vitro (DIV) (6). When cultures at 8 DIV (i.e., 5–7 days before the beginning of myelination) were treated with TTX for 2 or 4 days, the number of myelinated fibers at 18 to 21 DIV was decreased by 83 or 87% (Fig. 1A). Neither the number of oligodendrocytes nor the number of neuronal cell bodies were affected by the TTX treatment, and no evidence of oligodendroglial or neuronal injury could be detected at the ultrastructural level by electron microscopy. A treatment with TTX for only 2 days, at either 9 or 12 DIV, resulted at 21 DIV in 61 % and 98% decreases, respectively, in the number of myelinated fibers. The inhibitory effect of TTX depended, therefore, on the developmental state of the oligodendrocytes and neurons. It was most pronounced when the toxin was added just before the onset of myelination and was only transient: indeed, when parallel cultures were examined at 28 DIV, the number of myelinated segments was similar in the TTX-treated and control cultures. Similarly, when TTX was added to the culture at 18–21 DIV and the preparations analyzed at 28 DIV, no significant effect of the toxin was observed.
Fig. 1.

Tetrodotoxin (TTX) inhibits the onset of myelination A, Co-cultures of neurons and oligodendrocyte progenitors were established from cereberal hemispheres of 15-day-old mouse fetuses TTX (10−6 M) was added to the culture medium at 8 days in vitro (DIV) and maintained for 2 or 4 days, as indicated Cultures at 18 to 21 DIV were immunolabeled with an anti-myelin basic protein (MBP) antibody The total number of MBP + myelinated mternodes per coverslip was determined Values are the mean ± SEM of 8 different experiments Significant differences between control specimens and TTX-treated samples are indicated **, P < 0.02 two tailed unpaired Student’s t-test. B, An intraocular injection of TTX was performed in 4-day-old (P4) mice and the number of myelin-forming MBP+ oligodendrocytes as seen by epifluorescence microscopy m whole mounts of optic nerve were evaluated at P6. The total number of myelin-forming MBP+ cells was determined in TTX-treated and sham-injected control optic nerves, from the emergence of the optic canal to the optic chiasm Results obtained in 12 sham and 10 TTX-injected animals (four separate experiments) are expressed as the mean ± SEM, and significant differences between control specimens and TTX-treated samples are indicated :*, P < 0 05, two-tailed unpaired Student’s t-test) (Modified from Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, et al Induction of myelination in the central nervous system by electrical activity Proc Natl Acad Sci U S A 1996,93 9887-92. Copynght © 1996 the National Academy of Sciences, U.S.A. Used with permission.)
The effect of TTX on myelination could be caused either by the blockade of action potentials or by a change in the polarity of the axonal membrane as a result of its binding of TTX. To discriminate between these two possibilities, K+ was added to the culture medium either alone or together with TTX. Addition of K+ was not only unable to counteract the effect of TTX, it inhibited myelination itself, suggesting that the blockade of electrical activity, and not the changes in axonal membrane polarity, was responsible for the observed inhibition of myelination.
If blockade of the neuronal Na+ channels inhibits myelination, stimulation of neuronal activity by opening the channels should increase myelination. This can be achieved with the highly selective Na+ channel activator α-scorpion toxin (α-ScTX), which has been shown to dramatically increase the duration and frequency of spontaneous action potentials by slowing Na+ channel inactivation, without any effect on channel activation or resting membrane potential (38). Indeed, when cultures at 8 DIV were treated for 2 days with α-ScTX, the number of myelinated segments observed 10 days later increased by a factor of 2.4, compared with control cultures, without a significant effect on the number of oligodendrocytes. Longer exposures (4–6 days) to α-ScTX did not further increase the number of myelinated fibers.
To confirm the above in vitro findings, the effect of electrical activity on induction of myelination in the optic nerve was analyzed in situ. The time course of myelination in the optic nerve was established: nonmyelinating myelin basic protein (MBP; a component of myelin and mature oligodendrocytes) expressing oligodendrocytes were first seen on the retinal portion of the optic nerve at P4. These cells have very rich arborizations, reminiscent of the typical “sun-like” mature oligodendrocytes observed in culture. Myelinating oligodendrocytes were first detected at P6. They could easily be distinguished from nonmyelinating oligodendrocytes by their morphology. They had a reduced number of processes that were aligned along the neighboring axons to form myelin sheaths. Myelin-forming cells were also myelin oligodendrocyte glycoprotein positive (MOG+; a component of myelin and myelinating oligodendrocytes), which, in addition to the morphological criteria, distinguished them unambiguously from nonmyelinating oligodendrocytes (39). When TTX was injected into the right intravitreous space at P4 and the optic nerve examined at P6, there was no statistically significant modification in the total number of MBP+ oligodendrocytes, whereas myelinating oligodendrocytes were decreased by 75%, suggesting that electrical activity affected the onset of myelination (Fig. 1 B). This effect of TTX could not be attributed to axon damage, as shown by electron microscopic examination of the injected optic nerve. Injection of TTX at P5 did not significantly decrease the percentage of myelinating oligodendrocytes at P7. These results suggest that in situ as in vitro, electrical activity affects the myelination process only within a narrow time frame.
These results suggest that neuronal Na+ channels, hence electrical activity, are involved in myelination of the optic nerve. Interestingly, at the time when myelination starts, the electrical activity of retinal ganglion cells changes from a transient to a repetitive pattern of firing (40, 41). A similar correlation between impulse activity and initiation of myelination is evident in the medial forebrain bundle. Nigrostriatal fibers en route to the caudate nucleus and putamen are in close contact with myelinated fibers, and thus in the vicinity of mature myelin-forming oligodendrocytes, yet these fibers are not myelinated. It is of note, however, that nigrostriatal fibers are the axons from dopaminergic cells in the pars compacta, which are electrically silent (3 to 4 action potentials per second) most of the time.
Impulse Activity in Schwann Cell Myelination of PNS Axons
In contrast to the many studies of myelination by oligodendrocytes in the CNS, the possible effects of action potentials on myelination by Schwann cells in the PNS has been less investigated. The myelination process, anatomical structure, and biochemical composition of myelin in the PNS and CNS differ markedly. Although Schwann cells in culture (42) or introduced into the CNS can myelinate central axons (43, 44), different intrinsic and extrinsic factors probably regulate myelination by oligodendrocytes in the CNS and Schwann cells in the PNS. Evidence for this is provided by the observation that Schwann cells require contact with axons to initiate myelination (28–32), but myelin is elaborated by oligodendrocytes in culture in the absence of neuronal influence (45, 46). How impulse activity in the axon might be sensed by the Schwann cell and what molecular mechanism would be responsible for regulating myelination remain to be determined.
Regulation of Neuronal Gene Expression by Action Potentials
Work in the laboratory of author RDF on regulation of gene expression in neurons by different patterns of action potentials (47, 48) suggested one possible way in which myelination might be influenced by the firing pattern of an axon. These studies had shown that the expression of specific neural cell adhesion molecules (CAMs), known to have an important function in the myelination process, could be regulated by appropriate patterns of electrical activity in mouse dorsal root ganglion (DRG) neurons in vitro. Using a cell culture dish equipped with platinum electrodes for stimulating mouse DRG axons, they observed that the expression of calcium-dependent (47) and -independent (48) CAMs were regulated at the level of gene transcription by different frequencies of axonal firing. One of these CAMs, L1, has been shown to have a critical role in initiation of myelination (49, 50). When homophilic binding between L1 molecules on DRG axons and Schwann cells is blocked with L1 antibodies, early ensheathement and the initiation of myelination are blocked. This might provide a molecular mechanism linking appropriate firing patterns to myelination of these axons, but a number of uncertainties would potentially undermine this hypothesis. Electrical stimulation decreases the amount of L1 in the axonal membrane, but it does not eliminate L1-mediated binding to the extent that saturating concentrations of L1 antibodies would. In addition, transgenic mice lacking L1 develop normal myelinated axons, although moderate abnormalities affecting the nonmyelinating Schwann cell interaction with axons were detected (51).
Effects of Electrical Stimulation on Myelination of DRG Axons by Schwann Cells
To address this question, DRG neurons from fetal mice at 13.5 days of development were added to the side compartments of three-part cell culture chambers and cultured for 3 weeks (52). This period of time is sufficient for axons to grow under the barrier separating the side and central compartments. This allows stimulation of the axons that traverse the central and side compartments through extracellular electrodes on opposite sides of the barrier (53). An advantage of this preparation is that DRG neurons in culture do not fire action potentials spontaneously, and they respond to a brief electrical stimulus with a single action potential. This model system thus allows precise control of the pattern of impulse activity in cultured neurons for chronic periods, without depolarizing glial cells.
Schwann cells, derived from the sciatic nerve of postnatal mice, were added to 3-week-old cultures and permitted to associate with the axons for 24 hours. This period enabled Schwann cells to form stable contacts with axons and carry out early, premyelinating events, such as recognition and adhesion, before introducing the effects of stimulation. This also models the progression of events during embryonic development of DRG neurons in vivo (54, 55), in that Schwann cells become associated with DRG axons before the neurons develop spontaneous impulse activity. After DRG axons reach the subepidermis, they begin to fire spontaneously at low frequency. Later in development, as sensory end-organs become functional, the frequency of firing increases and becomes phasic, and Schwann cells begin to myelinate the large-diameter axons.
One day after co-culturing Schwann cells with the DRG neurons, cultures were stimulated at a rate of either 1 action potential every 10 sec (0.1Hz) or 1 action potential every sec (1Hz) for 5 days. These two frequencies resemble the different firing frequencies that are seen during early, premyelinating periods and late myelinating periods of development. Moreover, our previous work indicated that L1 levels in neurons should be reduced after 5 days stimulation in axons firing at 0.1Hz, but not on axons stimulated at 1 Hz (47, 48). Myelination was then initiated by the addition of ascorbic acid (56), a component in the culture medium that stimulates myelination. After 12 to 14 days, myelinated axons were stained with either Sudan black or antibodies against MBP and counted. Cultures were also examined by electron microscopy, which confirmed the formation of normal compact myelin. The results showed that although the ultrastructure of myelin was normal in stimulated and unstimulated cultures, only one third as many myelin profiles were present on axons stimulated at 0.1Hz, compared with either unstimulated controls or axons stimulated at 1 Hz (52) (Fig. 2).
Fig. 2.
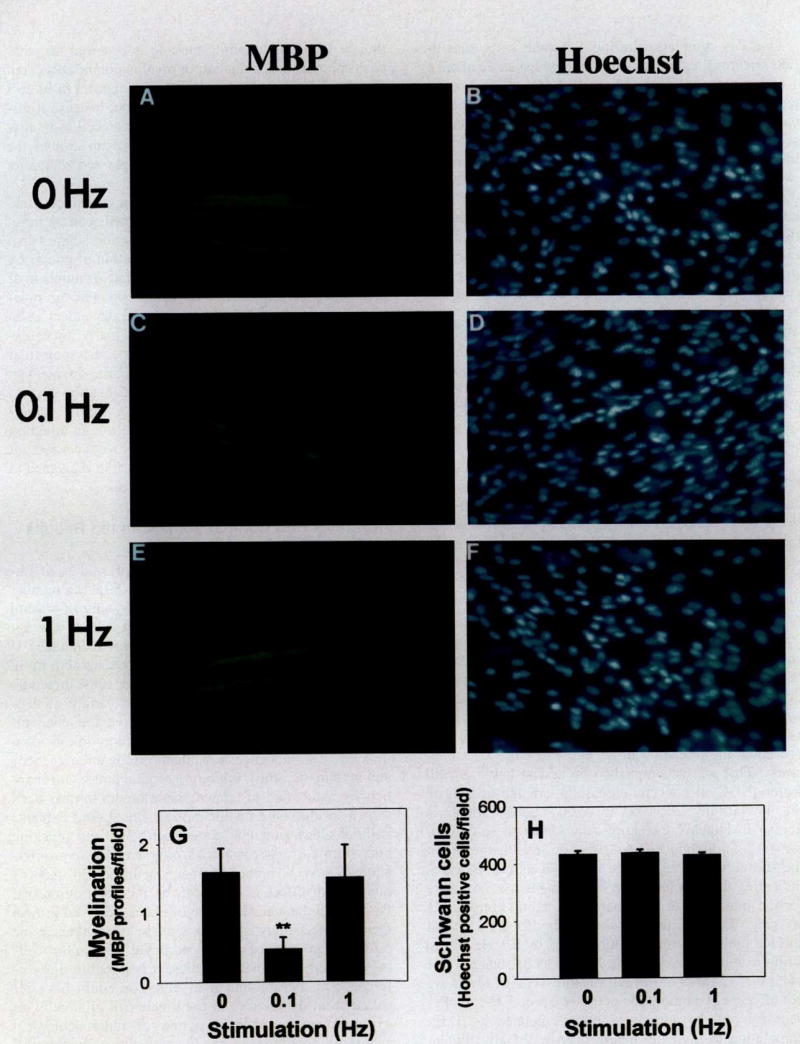
Myelination of dorsal root ganglion (DRG) axons is inhibited by 0.1 Hz electrical stimulation. Myelin profiles were identified by immunocytochemical staining for myelin basic protein (MBP) in cultures stimulated at 0 (A), 0.1 (C), and 1 (E) Hz for 5 days. Hoechst’s nuclear stain was used to count the number of Schwann cells in each preparation (B, D, F). G, The number of MBP- positive myelin profiles was significantly lower in cultures stimulated at 0.1 Hz. versus 0 or 1 Hz, n = 44 experiments; **, P < 0.001. H, The total number of Schwann cells was not significantly different in stimulated or unstimulated cultures. Scale bars, 50 μm. (Reprinted from Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci 1998;15: 9303–11. Copyright © 1998 by the Society for Neuroscience. Used with permission.)
The data provide no support for the hypothesis that the difference in number of myelinated axons was caused by differences in the number of Schwann cells in cultures stimulated at these different frequencies. The total number of Schwann cells in each condition was not significantly different, and the mitotic rate of the Schwann cells, measured using BrdU incorporation into mitotic nuclei, was not different in stimulated or unstimulated cultures.
A number of secreted or cell surface molecules might contribute to the reduced myelination after stimulation at 0.1Hz, but the correlation with the stimulus frequency that lowers L1 expression in DRG neurons is consistent with the involvement of this CAM. To test this hypothesis, stimulation was performed under conditions that prevented the reduction in L1 caused by 0.1 Hz stimulation. This was accomplished by adding nerve growth factor (NGF) at concentrations high enough to activate the low-affinity receptor (50–200 ng/ml), which is known to increase L1 expression. Under these circumstances, stimulation had no effect on myelination when the stimulus-induced change in L1 levels was blocked, indicating that the reduction in L1 levels was necessary for the inhibition of myelination on axons firing at 0.1 Hz (Fig. 3). It is possible that other diffusible or cell surface molecules may be modulated by 0.1 Hz stimulation to inhibit myelination, but evidence suggests that two other CAMs are not responsible. NCAM levels are not affected by stimulation at either 0.1 or 1 Hz in DRG neurons, and N-cadherin is down-regulated by 1 Hz stimulation to a greater extent than by 0.1 Hz stimulation, but this frequency had no effect on myelination.
Fig. 3.

Activity-dependent regulation of myelination requires down-regulation of the cell adhesion molecule L1 in dorsal root ganglion (DRG) neurons A, L1 mRNA levels were compared in DRG neurons and Schwann cells (SC) using reverse-transcnption/polymerase chain reaction (PCR). Stimulation at a frequency of 0.1 Hz for 5 days significantly lowered L1 expression in DRG neurons (136 base-pair [bp] PCR product, lane 1 vs lane 2), but stimulation at 1 Hz had no effect (lane 3 vs lane 1) Schwann cells express a short-splice isoform of L1 mRNA (124 bp PCR product), which was not altered by stimulation. B, Stimulation at 0.1 Hz had no effect on myelination when the stimulus-induced change in L1 levels was blocked by adding 50 ng/ml nerve growth factor. C, The down-regulation of L1 mRNA (136 bp) levels produced by 0 1 Hz stimulation was prevented by the addition of 50 ng/ml NGF during stimulation, which is known to upregulate L1 expression (Reprinted from Stevens B, Tanner S, Fields RD Control of myelination by specific patterns of neural impulses J Neurosci 1998;15 9303–11 Copynght © 1998 by the Society for Neuroscience. Used with permission.)
The reduced number of myelinated profiles on axons stimulated at low frequency is most likely a result of inhibition of the initiation phase of myelination. Although L1-L1 homophilic binding is essential for early ensheathment and induction of myelination of DRG neurons by Schwann cells in culture, L1 appears to be less important after initiation of myelination, because it disappears from both the axon and Schwann cell soon after the Schwann cell makes a complete wrap around the axon (57). Other CAMs become expressed thereafter (58).
The results of these experiments show that myelination of peripheral axons by Schwann cells can be influenced by impulse activity in the axon and suggest that the effects are not mediated by stimulation of glia or by secondary effects on the proliferation rate or numbers of glia. Moreover, the effects of action potentials on myelination by Schwann cells seem to be dependent upon the frequency of firing in the axon. Finally, these experiments provided evidence for a specific molecular mechanism linking appropriate rates of firing in the axon to myelinating activity of Schwann cells; namely, down-regulation of axonal expression of the CAM L1, a molecule that is known to play an essential role in initiation of myelination. However, these studies were carried out in vitro, and interpretations of the possible relevance to development in vivo remain hypothetical.
Contrasts and Comparisons: Can the Results be Reconciled?
The apparently contradictory results of studies of impulse activity on myelination in the CNS raise a number of questions and might be explained by any of several factors. One methodological difficulty, which was appreciated by many researchers using these techniques, is that pharmacological treatments to modulate activity in neurons can have direct affects on other cells, including oligodendrocytes. Glia express a wide variety of neurotransmitter-gated and voltage-sensitive ion channels (59, 60). Modulation of ion channel activity in oligodendrocytes can influence both their proliferation (61–65) and myelination (66). Inhibition of glial potassium channels by incubation of tetraethylammonium ion has been shown to eliminate myelination in spinal cord explants without altering axonal conduction (27), and blocking potassium currents can inhibit oligodendrocyte proliferation (62–64). Another mechanism that could underlie this type of effect is suggested by the observation that the activity-dependent release of platelet-derived growth factor from astrocytes in optic nerve can influence the proliferation rate of oligodendrocyte progenitor cells (66). In some published studies, it is possible that differences in the amount of myelination could have resulted from differences in the number of glial cells, as a consequence of direct action of pharmacological agents on proliferation of glia, rather than on the myelination process itself. It is also possible that this confounding effect on glial cell numbers could obscure an effect of action potentials on myelination in some experiments.
Secondly, intraocular injections of TTX were effective in inhibiting myelination of optic nerve only during a narrow developmental time window (37). Injections of TTX on P4 reduced myelination, but injections on P5 did not. Furthermore, blocking impulse activity had only a transient effect on myelination that was erased within about 1 week. This critical window of sensitivity to TTX might not have been sampled in some studies that report no effect of TTX on myelination of optic nerve. Similarly, the molecular mechanism implicated in the activity-dependent regulation of myelination in DRG neurons would only apply during the period when myelination is initiated, because L1 is not expressed after myelination has begun. An important consideration with respect to this critical window is that myelination of the optic nerve proceeds mostly with a spatial/temporal gradient from the retina to the chiasm, but differentiation of oligodendrocytes (including expression of myelin proteins) progresses with the reverse gradient (from the chiasm to the retina) (21). Therefore, the effective time point for TTX injections could differ depending on the anatomical location along the optic nerve examined in various studies and might be different depending on whether ultrastructural or biochemical analysis methods were used. Differences between the results of Collelo et al. (21) and Demerens et al. (37) may be attributed to differences in the experimental approaches used. Future experiments to reconcile these differences may be helpful. It is none-theless important to emphasize that an extrinsic influence on myelination, even if it is limited to the phase of development in which myelination is initiated, could provide an important influence on the developing structure and function of myelinated axons.
The results from the Zalc and Fields laboratories are compatible with the conclusion that impulse activity in the axon can have a significant influence on myelination, but there are some interesting differences between these two studies. The results of intraocular injection of TTX suggest that action potential activity promotes myelination in the optic nerve; indeed, the action potential activity seems to be a requisite trigger for myelination in that system. In the studies of DRG neurons, this is not so; myelination is normal in the absence of impulse activity and in axons firing at 1 Hz. Secondly, where impulse activity did influence myelination of DRG neurons by Schwann cells (i.e., on axons firing at 0.1 Hz), the effect was inhibitory.
The frequency-specific effects of impulse activity in the studies on DRG neurons may help reconcile some of the apparently contradictory results using pharmacological agents to stimulate or block action potentials in the CNS. Chronic depolarization with KCl does not reduce L1 expression measurably (47); therefore, chronic depolarization would not be expected to affect processes involved in myelination that are dependent upon L1. The particular frequencies of impulse activity influencing myelination may differ for different types of neurons with other characteristic firing patterns, or at different stages in development. Myelination is a multifaceted process, and different frequencies of impulse activity might affect different signaling pathways between axons and glia or have differential effects on the multiple processes involved in myelination, including cell proliferation, differentiation, cell-cell recognition, changes in motility, morphology, and gene expression.
It is interesting that the effects of impulse activity can be inhibitory when applied at appropriate frequencies. In DRG neurons, frequencies of impulse activity similar to those during the myelinating phase of development are not inhibitory to myelination, but low-frequency firing, resembling the pattern of impulse activity during pre-myelinating phases of development, inhibits myelination significantly. This suggests that initiation of myelination may be regulated by both positive and negative factors. Myelination in the CNS is different in many respects, but the results with Schwann cells raise the possibility that certain patterns of impulse activity could be inhibitory for myelination in the CNS and that activity-dependent regulation of an L1-like CAM could be involved in regulating myelination by oligodendrocytes in the CNS.
Acknowledgments
We thank S. G. Waxman for important contributions to the manuscript, B. Stevens and C. Lubetzki for critical comments on the manuscript.
References
- 1.Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–8. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- 2.Meyersburg HA, Post RM. An holistic developmental view of neural and psychological processes: a neurobiologic-psychoanalytic integration. Br J Psychol. 1979;135:139–55. doi: 10.1192/bjp.135.2.139. [DOI] [PubMed] [Google Scholar]
- 3.Travis F. Cortical and cognitive development in 4th, 8th and 12th grade students. The contribution of speed of processing and executive function to cognitive development. Biol Psychol. 1998;48:37–56. doi: 10.1016/s0301-0511(98)00005-2. [DOI] [PubMed] [Google Scholar]
- 4.McDonald WI. The pathophysiology of multiple sclerosis. In: McDonald WI, Silbergerg DH, editors. Multiple sclerosis. London: Butterworth; 1986. pp. 112–34. [Google Scholar]
- 5.Steck AJ, Schaeren-Wiemers N, Hartung HP. Demyelinating inflammatory neuropathies, including Guillain-Barre syndrome. Curr Opin Neurol. 1998;11:311–8. doi: 10.1097/00019052-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Lubetzki C, Demerens C, Anglade P, Villarroya H, Frankfurter A, Lee VM-Y, et al. Even in culture, oligodendrocytes myelinate solely axons. Proc Natl Acad Sci U S A. 1993;90:6820–4. doi: 10.1073/pnas.90.14.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rushton WAH. A theory of the effects of fibre size in medullated nerve. J Physiol (Lond) 1951;115:101–22. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiley-Livingston CA, Ellisman MA. Development of axonal membrane specializations defines nodes of Ranvier and precedes Schwann cell myelin elaboration. Dev Biol. 1980;79:334–55. doi: 10.1016/0012-1606(80)90120-7. [DOI] [PubMed] [Google Scholar]
- 9.Waxman SG, Foster RE. Development of the axon membrane during differentiation of myelinated fibres in spinal nerve roots. Proc R Soc Lond B Biol Sci. 1980;209:441–6. doi: 10.1098/rspb.1980.0105. [DOI] [PubMed] [Google Scholar]
- 10.Black JA, Foster RE, Waxman SG. Rat optic nerve: freeze-fracture studies during development of myelinated axons. Brain Res. 1982;250:1–10. doi: 10.1016/0006-8993(82)90948-9. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand C, Waxman SG. Postnatal differentiation of rat optic nerve fibers: electron microscopic observations on the development of nodes of Ranvier and axoglial relations. J Comp Neurol. 1984;224:25–37. doi: 10.1002/cne.902240103. [DOI] [PubMed] [Google Scholar]
- 12.Waxman SG. Axon-glial interactions: building a smart nerve fiber. Curr Biol. 1997;7:R406–10. doi: 10.1016/s0960-9822(06)00203-x. [DOI] [PubMed] [Google Scholar]
- 13.Sugihara I, Lang EJ, Llinas R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol (Lond) 1993;470:243–71. doi: 10.1113/jphysiol.1993.sp019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett MVL. Electric organs. Annu Rev Physiol. 1970;32:471–528. doi: 10.1146/annurev.ph.32.030170.002351. [DOI] [PubMed] [Google Scholar]
- 15.Waxman SG, Pappas GD, Bennett MVL. Morphological correlated of functional differentiation of nodes of Ranvier along single fibers in the neurogenic electric organ of the knife fish Stenarchus. J Cell Biol. 1972;53:210–24. doi: 10.1083/jcb.53.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanford LR. Conduction velocity variations minimize conduction time differences among retinal ganglion cell axons. Science. 1987;238:358–60. doi: 10.1126/science.3659918. [DOI] [PubMed] [Google Scholar]
- 17.Waxman SG. Closely spaced nodes of Ranvier in the teleost brain. Nature. 1970;227:283–4. doi: 10.1038/227283a0. [DOI] [PubMed] [Google Scholar]
- 18.Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function: a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–6. [PubMed] [Google Scholar]
- 19.Omlin FX. Optic disc and optic nerve of the blind cape mole-rat (Georychus capensis): a proposed model for naturally occurring reactive gliosis. Brain Res Bull. 1997;44:627–32. doi: 10.1016/s0361-9230(97)00283-9. [DOI] [PubMed] [Google Scholar]
- 20.Tauber H, Waehneldt TV, Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 1980;16:235–8. doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- 21.Colello RJ, Devey LR, Imperato E, Pott U. The chronology of oligodendrocyte differentiation in the rat optic nerve: evidence for a signaling step initiating myelination in the CNS. J Neurosci. 1995;15:7665–72. doi: 10.1523/JNEUROSCI.15-11-07665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crespo D, Verduga R, Villegas F, Fernandez-Viadera C. Dimorphic myelin in the rat optic nerve as a result of retinal activity blockage by tetrodotoxin during early postnatal period. Histo Histopathol. 1995;10:289–99. [PubMed] [Google Scholar]
- 23.Colello RJ, Pott U. Signals that initiate myelination in the developing mammalian nervous system. Mol Neurobiol. 1997;15:83–100. doi: 10.1007/BF02740617. [DOI] [PubMed] [Google Scholar]
- 24.Hayes WP, Meyer RL. Impulse blockade by intraocular tetrodotoxin during optic regeneration in goldfish: HRP-EM evidence that the formation of normal numbers of optic synapses and the elimination of exuberant optic fibers is activity independent. J Neurosci. 1989;9:1414–23. doi: 10.1523/JNEUROSCI.09-04-01414.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moor CL, Kalil R, Richards W. Development of myelination in optic tract of the cat. J Comp Neurol. 1976;165:125–36. doi: 10.1002/cne.901650202. [DOI] [PubMed] [Google Scholar]
- 26.Fukui Y, Hayashaka S, Bedi KS, Ozaki HS, Takeuchi Y. Quantitative study of the development of the optic nerve in rats reared in the dark during early postnatal life. J Anat. 1991;174:37–47. [PMC free article] [PubMed] [Google Scholar]
- 27.Shrager P, Novakovic SD. Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Dev Brain Res. 1995;88:86–7. doi: 10.1016/0165-3806(95)00081-n. [DOI] [PubMed] [Google Scholar]
- 28.Aguayo AJ, Charron L, Bray GM. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976;5:565–73. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- 29.Owens GC, Bunge RP. Evidence for an early role for myelin-associated glycoprotein in the process of myelination. Glia. 1989;2:119–28. doi: 10.1002/glia.440020208. [DOI] [PubMed] [Google Scholar]
- 30.Jessen KR, Mirsky R. Schwann cell precursors and their development. Glia. 1991;4:185–94. doi: 10.1002/glia.440040210. [DOI] [PubMed] [Google Scholar]
- 31.Scherer SS, Wang DY, Kuhn R, Lemke G, Wrabetz L, Kamholtz J. Axons regulate Schwann cell expression of the POU transcription factor SCIP. J Neurosci. 1994;14:1930–1942. doi: 10.1523/JNEUROSCI.14-04-01930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, et al. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–96. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 33.Lubetzki C, Demerens C, Zalc B. Signaux axonaux et myélino-génèse dans le système nerveux central. Medecine/Science. 1997;13:1097–105. [Google Scholar]
- 34.Althaus HH, Montz H, Neuhoff V. Isolation and cultivation of mature oligodendroglia. Naturwissench. 1984;71:309–15. doi: 10.1007/BF00396614. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 36.Macklin WB, Weill CL, Deminger PL. Expression of myelin proteolipid and basic protein mRNAs in culture cells. J Neurosci Res. 1986;16:203–17. doi: 10.1002/jnr.490160118. [DOI] [PubMed] [Google Scholar]
- 37.Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–92. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dargent B, Couraud F. Down-regulation of voltage-dependent sodium influx in developing neurons. Proc Natl Acad Sci U S A. 1990;87:5907–11. doi: 10.1073/pnas.87.15.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solly S, Thomas JL, Monge M, Demerens G, Lubetzski C, Gardinier MV, et al. Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia. 1996;18:39–48. doi: 10.1002/(SICI)1098-1136(199609)18:1<39::AID-GLIA4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Rörig B, Grantyn R. Glutamatergic and GABAergie synaptic currents in ganglion cells from isolated retinae of pigmented rats during postnatal development. Dev Brain Res. 1993;74:98–110. doi: 10.1016/0165-3806(93)90088-r. [DOI] [PubMed] [Google Scholar]
- 41.Skaliora I, Scobey RP, Chalupa LM. Prenatal development of excitability in cat retinal ganglion cells-action potentials and sodium currents. J Neurosci. 1993;3:313–23. doi: 10.1523/JNEUROSCI.13-01-00313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahr M, Hopkins JM, Bunge RP. In vitro myelination of regenerating adult rat retinal ganglion cell axons by Schwann cells. Brain Res. 1991;574:178–92. doi: 10.1002/glia.440040512. [DOI] [PubMed] [Google Scholar]
- 43.Felts PA, Smith KJ. Conduction properties of central nervous fibers remyelinated by Schwann cells. Brain Res. 1992;574:178–92. doi: 10.1016/0006-8993(92)90815-q. [DOI] [PubMed] [Google Scholar]
- 44.Duncan ID, Hoffman RL. Schwann cell invasion of the central nervous system of the myelin mutants. J Anat. 1997;190:35–49. doi: 10.1046/j.1469-7580.1997.19010035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradel EJ, Prince FP. Cultured rat oligodendrocytes elaborate myelin in the absence of neurons. J Neurosci Res. 1983;9:381–92. doi: 10.1002/jnr.490090404. [DOI] [PubMed] [Google Scholar]
- 46.Rome LH, Bullock PN, Chiappelli F, Cardwell M, Adinoff AM, Swanson D. Synthesis of myelin-like membrane by oligodendrocytes in culture. J Neurosci Res. 1986;15:49–65. doi: 10.1002/jnr.490150106. [DOI] [PubMed] [Google Scholar]
- 47.Itoh K, Ozaki M, Stevens B, Fields RD. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM and L1 by distinct patterns of action potentials. J Neurobiol. 1997;33:735–48. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 48.Itoh K, Stevens B, Schachner M, Fields RD. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–72. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- 49.Seilheimer B, Persohn E, Schachner M. Antibodies to the L1 adhesion molecule inhibit Schwann cell ensheathment of neurons in vitro. J Cell Biol. 1989;107:341–51. doi: 10.1083/jcb.109.6.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood PM, Schachner M, Bunge RP. Inhibition of Schwann cell myelination in vitro by antibody to the L1 adhesion molecule. J Neurosci. 1990;10:3635–45. doi: 10.1523/JNEUROSCI.10-11-03635.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–9. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- 52.Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;15:9303–11. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fields RD, Yu C, Neale EA, Nelson PG. Chronic electrical stimulation of multicompartment cell cultures. In: Kettenman H, Grantyn R, editors. Practical electrophysiological methods. New York: Wiley-Liss; 1992. [Google Scholar]
- 54.Fitzgerald M. Spontaneous and evoked activity of fetal primary afferents in vivo. Nature. 1987;326:603–7. doi: 10.1038/326603a0. [DOI] [PubMed] [Google Scholar]
- 55.Fields RD. Effects of ion channel activity on development of dorsal root ganglion neurons. J Neurobiol. 1998;37:158–70. doi: 10.1002/(sici)1097-4695(199810)37:1<158::aid-neu12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 56.Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105:1023–33. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martini R, Schachner M. Immunoelectron miroscopic localization of neural adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. Cell Biol. 1986;103:2439–48. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration. J Neurocytol. 1994;23:1–28. doi: 10.1007/BF01189813. [DOI] [PubMed] [Google Scholar]
- 59.Ranson BR, Orkand RK. Glial-neuronal interactions in non-synaptic areas of the brain: studies in the optic nerve. Trends Neurosci. 1996;19:352–8. doi: 10.1016/0166-2236(96)10045-x. [DOI] [PubMed] [Google Scholar]
- 60.Sontheimer H, Black JA, Waxman SG. Voltage-gated Na+ channels in glia: properties and possible functions. Trends Neurosci. 1996;19:325–31. doi: 10.1016/0166-2236(96)10039-4. [DOI] [PubMed] [Google Scholar]
- 61.Chiu SY, Wilson GF. The role of potassium channels in Schwann cell proliferation in Wallerian degeneration of explant rabbit sciatic nerves. J Physiol (Lond) 1989;408:199–222. doi: 10.1113/jphysiol.1989.sp017455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pappas CA, Ulrich N, Sontheimer H. Reduction of glial proliferation by K+ channel blockers is mediated by changes in pH. Neuroreport. 1994;6:193–6. doi: 10.1097/00001756-199412300-00049. [DOI] [PubMed] [Google Scholar]
- 63.Gallo V, Zhou JM, McBam CJ, Wright CJ, Knutson PL, Armstrong RCl. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–70. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knutson P, Ghiani CA, Zhou JM, Gallo V, McBain CJ. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in gligodendrocyte progenitor cells. J Neurosci. 1997;17:2669–82. doi: 10.1523/JNEUROSCI.17-08-02669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacFarlane SN, Sontheimer H. Electrophysiological changes that accompany reactive gliosis in vitro. J Neurosci. 1997;17:7316–29. doi: 10.1523/JNEUROSCI.17-19-07316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–60. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]


