Abstract
Previous research has examined neural responses to threatening facial expressions such as those displaying anger, fear, and disgust. Here, we examined neural responses to a different type of threatening facial expression that primarily signifies a threat to social connection, namely a “disapproving” facial expression. We hypothesized that neural responses to disapproving facial expressions would be moderated by individual differences in rejection sensitivity. Using functional magnetic resonance imaging (fMRI), we scanned participants while they viewed brief video clips of facial expressions depicting disapproval, anger, and disgust. As expected, all three expressions yielded bilateral amygdala activation relative to a resting baseline. Additionally, individuals who scored higher on a measure of rejection sensitivity exhibited greater dorsal anterior cingulate cortex activity in response to disapproving facial expressions, but not in response to anger or disgust facial expressions. Results suggest that, at the neural level, individuals high in rejection sensitivity may be more sensitive to facial expressions signaling potential rejection, but not to threatening facial expressions in general. Results also suggest that disapproving facial expressions convey a distinct type of threat and should be considered in future studies of socially threatening facial expressions.
Facial expressions are a powerful and efficient source of social information (Darwin, 1872/1998). They are a reflection of how we feel and they help us communicate these feelings to others. As highly social beings, we are constantly exposed to a variety of different facial expressions, each providing valuable information about our physical and social surroundings and how to respond appropriately to them (Darwin, 1872/1998; Ekman, 2003). Negative or threatening facial expressions, which signal to the target individual that “something is wrong” or that he or she is in some sort of danger, are especially potent. For example, an angry scowl can signal that we have crossed someone and should prepare to either fight or flee, and a grimace of disgust can warn us to steer clear of certain foods, helping us avoid food poisoning. Using tools such as functional magnetic resonance imaging (fMRI), we can examine the underlying neural responses to these negative facial expressions, and thus, deepen our understanding of how these expressions affect us.
Dozens of studies have investigated the brain's response to various threatening expressions, such as fear, anger, and disgust (fear: Morris et al., 1996; Phillips et al., 1997; Whalen et al., 1998, 2001; anger: Fitzgerald, Angstadt, Jelsone, Nathan, & Phan, 2006; Whalen et al., 2001; disgust: Fitzgerald et al., 2006; Phillips et al., 1997), with most studies finding activity in the amygdala,1 a limbic structure theorized to play a role in the processing of threatening as well as socioemotional information from faces (Adolphs, 2002, 2003; Adolphs, Baron-Cohen, & Tranel, 2002; Whalen et al., 1998, 2001). Numerous studies have also observed insula and prefrontal activity in response to these negative emotional cues (Britton, Taylor, Sudheimer, & Liberzon, 2006; Kesler-West et al., 2001; Kilts, Egan, Gideon, Ely, & Hoffman, 2003; Phillips et al., 1997, 1998; Sprengelmeyer, Rausch, Eysel, & Przuntek, 1998; see Murphy, Nimmo-Smith, & Lawrence, 2003; Phan, Wager, Taylor, & Liberzon, 2002; Wager, Phan, Liberzon, & Taylor, 2003, for reviews). Despite this wealth of research, there is another type of threatening facial expression whose neural signature has yet to be examined. To date, the neural response to an expression that specifically conveys social rejection or disapproval is unknown. Thus, the goal of this study was to use fMRI to examine the neural responses to disapproving facial expressions, how these responses may resemble or differ from responses to other previously studied threatening facial expressions, and how they may vary across individuals based on differences in rejection sensitivity.
What is a disapproving facial expression? A disapproving facial expression is an indication of a negative evaluation. It is a signal that the target has done or said something socially undesirable and, consequently, has potentially damaged a social connection. A disapproving facial expression can convey a qualitatively different message than other threatening facial expressions such as fear, anger, or disgust. Fear and anger expressions often communicate that the target may be in danger of physical harm, and disgust expressions typically signal that the target may be in danger of physical contamination. In fact, the primary evolved purpose of anger, fear, and disgust expressions is assumed to be to signal a threat to physical safety (Darwin, 1872/1998). In contrast, disapproving facial expressions only signify a threat to social connection and have no other connotation.
Of course, facial expressions, such as anger or disgust, which can convey the threat of physical harm, may also convey the threat of social harm. One factor that may determine whether an expression is interpreted as a physical threat, a social threat, or both, is the familiarity of the individual displaying the expression. When the individual is familiar, the target individual has additional information from past interactions to help process the facial expression. For example, if the individual has a history of violence, the target individual will likely perceive an anger expression as primarily a physical threat. If the individual is known to be a peaceful person, the anger expression may not be interpreted as signaling physical danger, but rather, as indicating a purely social threat. Additionally, if the individual is a close other (e.g., spouse, parent, friend), expressions of anger and disgust will likely be interpreted, to some extent, as a social threat capable of damaging the relevant relationship. On the other hand, if the individual is unfamiliar, it may be difficult to definitively eliminate the possibility of physical danger associated with an anger or disgust expression and thus may be adaptive to prepare for the possibility of physical harm. Thus, we suggest that, at least with unfamiliar others, disapproving facial expressions are more likely to be interpreted as purely social threats than other threatening expressions. In the present study, we focused exclusively on responses to expressions conveyed by unfamiliar others.
We operationalized a disapproving facial expression as one that involves raising one side of the upper lip, lowering the inner corners of the brow in a fashion similar to that displayed when expressing “confusion,” and slightly tilting or pulling the head backwards. Figure 1 shows a still frame from a video clip of a volunteer demonstrating a disapproving expression. The disapproving facial expressions used in our study are somewhat similar to the “contempt” faces described by Darwin (1872/1998) and Matsumoto and Ekman (2004) in that both convey dissatisfaction with some aspect of the target individual, and both involve a unilateral lip raise and sometimes a slight tilting or pulling back of the head. However, unlike the disapproval expressions we used, contempt expressions do not usually involve brow movement and are further described as involving a “wrinkling of the nose … commonly accompanied by a slight snort … half-closing [the] eyelids, or turning away …” (Darwin, 1872/1998, pp. 252–254). Additionally, contempt expressions may involve more disgust or hatred than disapproving facial expressions.
Figure 1.

Still frames from video clips of (from left to right) disapproval, anger, and disgust facial expressions. The video clips presented all facial expressions in full color.
Understanding the impact of disapproving facial expressions on targets has important consequences for mental and physical health outcomes. For example, social rejection has been shown to be associated with such negative outcomes as decrements in self-regulation (Baumeister, DeWall, Ciarocco, & Twenge, 2005), increases in negative affect, and increased physiological stress responses such as elevated blood pressure (Stroud, Tanofsky-Kraff, Wilfley, & Salovey, 2000) and cortisol (Dickerson & Kemeny, 2004). Facial expressions signaling potential social rejection may have similar effects. Additionally, while facial expressions such as fear and anger have been used to assess negative affective processes in several clinical disorders, including social phobia (Amir et al., 2005; Birbaumer et al., 1998; Phan, Fitzgerald, Nathan, & Tancer, 2006), posttraumatic stress disorder (Rauch et al., 2000), depression (Lawrence et al., 2004; Sheline et al., 2001), and borderline personality disorder (Donegan et al., 2003), disapproving facial expressions may be more relevant and potent for understanding clinical disorders that predominantly involve a sensitivity to negative social experiences (e.g., social phobia, depression).
Whether at the clinical or subclinical level, individuals likely differ in the extent to which they may be affected by cues of potential rejection, such as disapproving facial expressions. “Rejection sensitivity” refers to the extent to which an individual anxiously expects, readily perceives, and overacts to social rejection (Downey & Feldman, 1996). In other words, an individual high in rejection sensitivity is more likely to interpret ambiguous stimuli as rejecting, subsequently overreact, and consequently feel greater distress, compared to individuals low in rejection sensitivity (Downey & Feldman, 1996). Since rejection sensitivity can moderate social, cognitive, and behavioral responses to potentially socially threatening information, it follows that it may also moderate neural responses to cues of potential social rejection, such as disapproving facial expressions. Thus, we expected that individuals high in rejection sensitivity would be particularly sensitive to disapproving facial expressions at the neural level.
Although the neural response to disapproving facial expressions has not yet been examined, we predicted that certain neural regions would be involved based on related research. First, we expected to see amygdala activity in response to viewing disapproving facial expressions because the amygdala has been shown to be activated in response to a variety of other threatening facial expressions such as anger, fear, and disgust (Fitzgerald et al., 2006; Morris et al., 1996; Phillips et al., 1997; Whalen et al., 1998, 2001). However, because the threat displayed in a disapproving facial expression is fundamentally more social in nature than the threat conveyed by anger, fear, or disgust expressions, we also expected to find neural activity unique to the disapproving facial expressions. The dorsal anterior cingulate cortex (dACC) was a primary candidate. Prior work has shown that dACC is activated in response to an episode of social rejection and that the magnitude of dACC activity correlates with the magnitude of self-reported social distress (e.g., “I felt rejected”) following rejection (Eisenberger, Lieberman, & Williams, 2003; Eisenberger, Way, Taylor, Welch, & Lieberman, 2007). Based on this finding, it is possible that facial stimuli that indicate social rejection (i.e., disapproving expressions) may activate the dACC as well, either (a) because these expressions elicit social distress in the target and thus activate distress-related dACC activity or (b) because dACC activity may be sensitive to cues of social rejection, in addition to experiences of social rejection. Thus, we hypothesized that dACC would be responsive to disapproving facial expressions, but not to other threatening expressions. Most importantly, we hypothesized greater dACC activity in response to the disapproving facial expressions in individuals who are high in rejection sensitivity.
Here we present the findings of an fMRI study that supports the hypothesis that rejection-sensitive individuals show a greater “threat response” to disapproving facial expressions than non rejection-sensitive individuals. In this study, we scanned healthy participants while they viewed dynamic disapproval, anger, and disgust faces.2 We examined neural activity in response to the presentation of each of these expressions (i.e., main effects) as well as the activity that was correlated with participants' self-reported scores of rejection sensitivity.
Methods
Participants
Twenty-one participants (15 females; mean age = 27.10 years, SD = 9.04), who were recruited from a UCLA undergraduate psychology class and the UCLA community, were paid $30 to complete the study. Data from two participants (both female) were excluded due to excessive head movement during scanning, and therefore, main effects analyses included 19 participants. In addition, only 16 of these 19 participants (11 females) completed the self-report measure of rejection sensitivity and thus all regression analyses using this variable involved these 16 participants.
Stimuli
The stimuli consisted of brief, 3-second video clips of seven volunteers (four female) demonstrating anger, disgust, disapproving, and neutral3 facial expressions. The video clips were recorded by the experimenters using a Sony digital video recorder and edited on a Macintosh G4 iMac with iMovie software. The video clips were validated by having a separate group of participants (n = 17) rate them, using two different methods. First, participants were instructed to label the emotion or feeling conveyed by each facial expression using a free-response format. Subsequently, participants were instructed to identify the emotion or feeling conveyed by each facial expression using a forced-choice response sheet with the following options: anger, disgust, disapproval, sadness, confusion, and neutral. Using the free-response format, participants identified the expressions at a rate significantly higher than chance (84% correct overall; 85% correct for disapproval faces specifically). Examples of free responses (to the question “What emotion or feeling is being conveyed?”) that were coded as “disapproval” included phrases like: “disapproving,” “disappointed,” and “shouldn't have done that.” Examples of free responses that were coded as “anger” included “angry” and “mad.” Examples of free responses that were coded as “disgust” included “disgusted,” “repulsed,” and “grossed out.” Using the forced-choice method, participants accurately labeled the expressions at a level significantly higher than chance (86% correct overall; 79% correct for disapproval faces specifically; chance = 16.7%). These recognition rates are consistent with those observed for emotional expressions (i.e., anger, disgust, fear) in standardized stimulus sets (Biehl et al., 1997; Tottenham, Borscheid, Ellertsen, Marcus, & Nelson, 2002).
Procedure
To assess neural activity in response to viewing the dynamic facial expressions, participants viewed the video clips of these expressions within the fMRI scanner. Participants were fitted with LCD goggles that enabled them to see the stimuli presented on a Macintosh G4 Powerbook computer. Each participant viewed a total of eight blocks of video clips. This included two blocks each of anger, disgust, disapproval, and neutral facial expressions. Each block consisted of ten 3-second video clips of different individuals demonstrating the particular expression type for that block, for a total of 30 seconds per block. Participants were instructed to simply watch the video clips. Experimental blocks were separated by 10-second rest blocks, during which time, the participants were instructed to focus on a crosshair, thereby establishing a baseline comparison condition (“fixation”).
Outside the scanner, participants completed a self-report measure of rejection sensitivity—the Rejection Sensitivity Questionnaire (RSQ-Short Form; Downey & Feldman, 1996). This measure consisted of 8 items in which participants were given social scenarios (e.g., “You ask a friend to do you a big favor”) and then asked about the extent to which they would feel anxious about rejection (“How concerned or anxious would you be over whether or not your friend would do this favor?”) and the extent to which they would expect rejection (“I would expect that he/she would willingly do this favor for me”), given the scenario. Ratings of anxiety were made on a 6-point scale from 1 (very unconcerned) to 6 (very concerned). Ratings of expectation of rejection were made on a 6-point scale from 1 (very unlikely) to 6 (very likely). The RSQ-Short Form scores were obtained by multiplying the anxiety and expectation rating for each of the eight scenarios, and then taking the average of these eight products. Most participants completed the measure in the same session as the fMRI task, prior to being scanned; however, some completed the measure several weeks following the scanning session.
Image acquisition and analysis
Data were acquired on a Siemens Allegra 3T scanner at the UCLA Ahmanson-Lovelace Brainmapping Center. Head movements were restrained with foam padding and surgical tape that was attached to the goggles and scanner bed. High-resolution structural T2-weighted echo-planar images (spin-echo; TR = 5000 ms; TE = 33 ms; matrix size 128 × 128; 36 saggital slices; FOV = 20 cm; 3 mm thick, skip 1 mm) were acquired coplanar with the functional scan. One functional scan lasting 5 minutes and 30 seconds was acquired (echo planar T2*-weighted gradient-echo, TR = 3000 ms, TE = 25 ms, flip angle = 90°, matrix size 64 × 64, 36 axial slices, FOV = 20 cm; 3 mm thick, skip 1 mm).
The imaging data were analyzed using SPM'9 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Images for each participant were realigned to correct for head motion, normalized into a standard stereo-tactic space as defined by the Montreal Neurological Institute, and smoothed with an 8 mm Gaussian kernel, full width at half maximum, to increase the signal-to-noise ratio. The design was modeled using a boxcar function convolved with a canonical hemodynamic response function. Linear contrasts were used to assess neural activity during each of the experimental conditions (anger, disgust, disapproval) compared to the baseline rest condition (crosshair fixation), as well as during each experimental condition compared directly to the others (e.g., disapproval vs. anger). Random effects group analyses were computed using participants' individual contrast images.
To assess correlations between rejection sensitivity and neural activity in response to the disapproving expressions, self-report scores of the rejection sensitivity measure were entered as a regressor into a whole-brain random effects group analysis for the contrast comparing the disapproval condition to fixation. Similar whole-brain regression analyses were also completed for the contrasts comparing the anger condition to fixation and the disgust condition to fixation in order to assess correlations between rejection sensitivity and neural activity in response to the anger and disgust expressions.
To examine functional connectivity between those neural regions hypothesized a priori to be moderated by rejection sensitivity (i.e., dACC) and other regions associated with rejection sensitivity in response to disapproving faces, parameter estimates were extracted at the region of interest and entered as a regressor into a whole-brain random effects group analysis to reveal regions correlated with the region of interest.
To correct for multiple comparisons in the whole-brain analyses, we used an uncorrected p-value of .005 combined with a cluster size threshold of 10 voxels (Forman et al., 1995) for all limbic (e.g., amygdala, insula, ACC) and prefrontal cortical regions, as these regions have been shown to be involved when viewing emotional facial expressions (Fitzgerald et al., 2006; Morris et al., 1996; Phillips et al., 1997; Wager et al., 2003; Whalen et al., 1998, 2001). For all other regions, we used a p-value of .05, corrected, with a 10-voxel extent threshold. All coordinates are reported in Montreal Neurological Institute (MNI) format.
Results
Rejection sensitivity
The mean of participants' scores on the self-report measure of rejection sensitivity (M = 7.53) was slightly lower than the measure's reported mean (M = 9.69; Downey & Feldman, 1996). The observed standard deviation (SD = 3.05) was similar to the measure's reported standard deviation (SD = 3.07; Downey & Feldman, 1996). The reliability of this scale was strong (α = .73, standardized item α = .76)
Neural activations common to disapproval, anger, and disgust
As predicted and shown in Table 1, each condition (disapproval, anger, disgust), compared to fixation, resulted in significant activity in bilateral amygdala (p < .005, 10-voxels), similar to previous studies of neural activity to negative facial expressions (Fitzgerald et al., 2006; Morris et al., 1996; Philips et al., 1997; Whalen et al., 1998, 2001). In addition, each condition, compared to fixation, resulted in activity in several regions of the prefrontal cortex, including the medial prefrontal cortex (MPFC; Brodmann's area (BA) 10), dorsomedial prefrontal cortex (DMPFC; BA 8 & 9), ventromedial prefrontal cortex (VMPFC; BA 11), right and left ventrolateral prefrontal cortex (RVLPFC, LVLPFC; BA 47), and right and left inferior frontal gyrus (RIFG, LIFG; BA 45). Contrary to our prediction, however, there was no significant ACC activity (dorsal or rostral) in response to any of the conditions relative to fixation. Table 1 lists activations for regions found to be activated in response to all three types of facial expressions relative to fixation.
Table 1.
Common neural regions that showed increased activity during the viewing of each facial expression compared to fixation
| Disapproval
|
Anger
|
Disgust
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | x | y | z | t-statistic | x | y | z | t-statistic | x | y | z | t-statistic |
| Left Amygdala | −22 | −6 | −16 | 6.42 | −22 | −4 | −16 | 6.87 | −22 | −6 | −18 | 6.55 |
| Right Amygdala | 16 | −6 | −14 | 4.12 | 30 | 0 | −24 | 5.09 | 28 | −4 | −20 | 7.08 |
| DMPFC (BA 8) | −8 | 50 | 44 | 7.52 | −4 | 56 | 44 | 3.07 | −8 | 50 | 48 | 4.36 |
| DMPFC (BA 9) | −6 | 60 | 30 | 5.52 | −6 | 60 | 34 | 4.28 | −6 | 64 | 30 | 5.15 |
| −6 | 52 | 40 | 5.92 | |||||||||
| MPFC (BA 10) | −6 | 64 | 20 | 4.77 | −6 | 68 | 18 | 3.38 | −8 | 66 | 18 | 3.72 |
| −10 | 64 | 26 | 3.89 | −8 | 66 | 24 | 5.94 | |||||
| VMPFC | 2 | 44 | −20 | 6.00 | −2 | 44 | −20 | 5.26 | −2 | 46 | −22 | 4.42 |
| 2 | 50 | −20 | 5.92 | 2 | 46 | −20 | 5.26 | |||||
| LVLPFC | −46 | 28 | −4 | 5.89 | −52 | 40 | −2 | 3.19 | −46 | 28 | −4 | 3.38 |
| −40 | 38 | −14 | 3.63 | −32 | 26 | −20 | 4.43 | |||||
| −32 | 36 | −20 | 3.77 | |||||||||
| RVLPFC | 44 | 28 | −8 | 6.64 | 46 | 26 | −4 | 5.20 | 46 | 28 | −8 | 3.79 |
| 46 | 30 | −6 | 6.70 | 42 | 34 | −16 | 3.55 | |||||
| 46 | 50 | −12 | 3.96 | |||||||||
| 50 | 26 | −12 | 6.05 | |||||||||
| LIFG (BA 45) | −56 | 22 | 8 | 6.56 | −48 | 24 | 16 | 8.70 | −54 | 24 | 14 | 4.74 |
| RIFG (BA 45) | 56 | 22 | 20 | 7.52 | 56 | 22 | 8 | 3.09 | 56 | 28 | 10 | 3.54 |
| Occipital (BA 17/18)* | −8 | −98 | 6 | 16.52 | −14 | −98 | 12 | 16.60 | 12 | −100 | 10 | 22.22 |
Note: All activations: p < .005, uncorrected, 10-voxel extent threshold except *p < .05, corrected, 10-voxel extent threshold. BA = Brodmann's area.
Activations that differentiate disapproval from anger and disgust
Table 2 shows regions differentially active when comparing the disapproval condition directly with the anger and disgust conditions. Interestingly, there was significantly greater activity in the left amygdala during disapproval expressions than during anger expressions (p < .005, 10-voxels; see Figure 2). In addition, consistent with prior work on disgust (Phillips et al., 1997, 1998; Sprengelmeyer et al., 1998), there was significantly greater insula activity when subjects viewed the disgust faces, compared to when they viewed the disapproval faces (p < .005, 10-voxels; see Figure 3). Again, there were no significant differences in dACC activity when comparing the disapproval condition with the other conditions.
Table 2.
Regions that show differential neural activity while viewing disapproving facial expressions compared to other facial expressions
| Region | x | y | z | t-statistic |
|---|---|---|---|---|
| Disapproval > anger | ||||
| Left Amygdala | −22 | −6 | −12 | 4.39 |
| LVLPFC (BA 47) | −52 | 32 | −4 | 3.77 |
| RVLPFC (BA 47) | 48 | 26 | −14 | 4.14 |
| MTG (BA 21)* | 56 | −34 | 0 | 7.76 |
| Anger > disapproval | ||||
| MPFC | 12 | 62 | 2 | 3.77 |
| ROFC (BA 11/47) | 20 | 24 | −16 | 3.63 |
| Disapproval > disgust | ||||
| No significant activity | ||||
| Disgust > disapproval | ||||
| Insula | 38 | −18 | −2 | 4.12 |
| MPFC | 12 | 66 | 8 | 3.76 |
| RVLPFC (BA 11/47) | 28 | 26 | −20 | 4.24 |
| 28 | 30 | −12 | 4.16 | |
| LDLPFC (BA 9) | −36 | 42 | 36 | 3.69 |
Note: All activations: p < .005, uncorrected, 10-voxel extent threshold except *p < .05, corrected, 10-voxel extent threshold. BA = Brodmann's area; MTG = middle temporal gyrus; ROFC = right orbital frontal gyrus.
Figure 2.
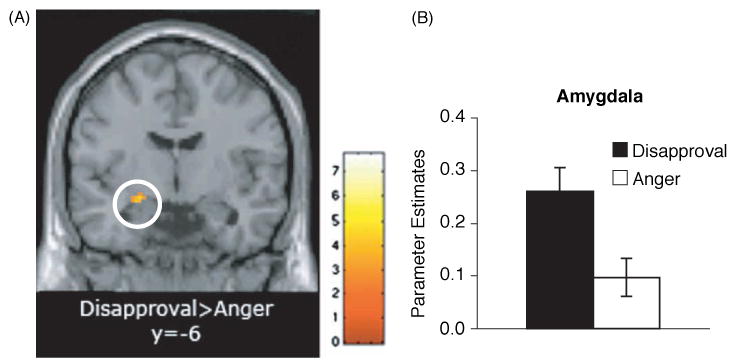
(A) Coronal slice (at y = −6); (B) bar graph showing greater amygdala activity in response to disapproval than anger (p < .005, 10-voxels).
Figure 3.
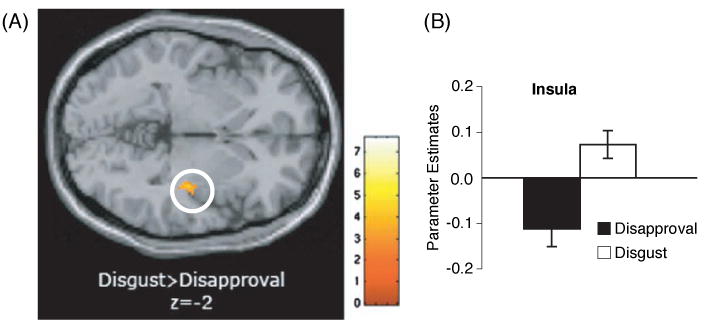
(A) Axial slice (at z = −2); (B) bar graph showing greater insula activity in response to disgust than disapproval (p < .005, 10-voxels).
Correlations with rejection sensitivity
Positive correlations
To examine correlations between rejection sensitivity and neural responses to the facial expressions, we regressed rejection sensitivity scores into whole-brain random effects group analyses, comparing each facial expression to fixation. There were no limbic or prefrontal regions that correlated positively with rejection sensitivity during the anger condition relative to fixation or during the disgust condition relative to fixation. In contrast, during the disapproval condition, relative to fixation, rejection sensitivity was positively correlated with activity in the dACC (r = .70, p < .005; see Table 3 and Figure 4) as well as with activity in RVLPFC and right dorsolateral prefrontal cortex (RDLPFC; see Table 3). Thus, as predicted, to the extent that an individual scored higher in rejection sensitivity, that individual exhibited increased activity in the dACC and other prefrontal regions in response to the disapproving facial expressions relative to fixation, but not in response to the anger or disgust facial expressions relative to fixation.
Table 3.
Neural regions that correlate with rejection sensitivity while viewing facial expressions compared to fixation
| Region | x | y | z | r | t-statistic |
|---|---|---|---|---|---|
| Positive correlation with RS during disapproval > fixation | |||||
| dACC | 0 | 24 | 30 | .68 | 3.43 |
| 8 | 30 | 22 | .66 | 3.31 | |
| 14 | 24 | 40 | .70 | 3.68 | |
| 14 | 32 | 24 | .65 | 3.18 | |
| 6 | 38 | 36 | .66 | 3.30 | |
| 16 | 26 | 42 | .69 | 3.54 | |
| RVLPFC | 26 | 22 | −18 | .75 | 4.24 |
| RDLPFC | 22 | 52 | 42 | .71 | 3.74 |
| Negative correlation with RS during disapproval > fixation | |||||
| SubACC/VMPFC | −4 | 26 | −12 | −.75 | 4.18 |
| Positive correlation with RS during anger > fixation | |||||
| No significant activity | |||||
| Negative correlation with RS during anger > fixation | |||||
| dACC | 0 | 4 | 32 | −.68 | 3.44 |
| −8 | 6 | 48 | −.70 | 3.69 | |
| SubACC/VMPFC | 8 | 26 | −14 | −.75 | 4.18 |
| MPFC (BA 10) | 2 | 46 | 20 | −.65 | 3.24 |
| Left Amygdala | −20 | −2 | −12 | −.68 | 3.46 |
| Positive correlation with RS during disgust > fixation | |||||
| No significant activity | |||||
| Negative correlation with RS during disgust > fixation | |||||
| Right Amygdala | 22 | −8 | −10 | −.64 | 3.08 |
Note: All activations p < .005, uncorrected, 10-voxel extent threshold. BA = Brodmann's area; RS = rejection sensitivity.
Figure 4.
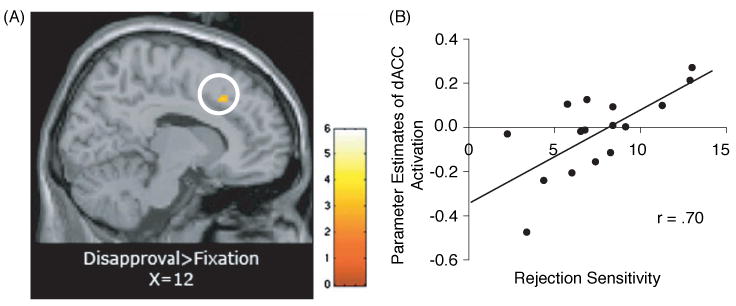
(A) dACC activity (14, 24, 40) in response to disapproval expressions compared to fixation that correlates positively with self-reported rejection sensitivity scores (r = .70; p < .005, 10-voxels); (B) scatterplot showing the relationship between rejection sensitivity scores and dACC activity (14, 24, 40) in response to disapproving expressions compared to fixation.
To further explore the specificity of the relationship between rejection sensitivity and dACC activity in response to the disapproving expressions, we completed two sets of post hoc analyses. First, we lowered our statistical threshold to further examine the relationship between rejection sensitivity and dACC activity in response to the anger and disgust expressions relative to fixation. Even when the statistical threshold was lowered to p < .3, there were no regions of dACC that were positively correlated with rejection sensitivity in response to the anger faces relative to fixation or in response to the disgust faces relative to fixation. Next, we completed additional analyses examining the relationship between dACC activity and rejection sensitivity in response to the disapproving expressions compared directly with anger and disgust faces. As shown in Table 4, using dACC ROIs based on the activity correlating with rejection sensitivity in response to the disapproving faces compared to fixation, rejection sensitivity was also positively correlated with dACC activity in response to the disapproving facial expressions when compared directly with either anger or disgust expressions (p < .05), suggesting that rejection sensitivity correlated with dACC activity to a significantly greater extent while viewing disapproving faces than while viewing disgust or anger faces.
Table 4.
dACC ROI activity that is positively correlated with rejection sensitivity while viewing disapproving facial expressions compared directly to anger and disgust facial expressions
| Region | x | y | z | r | t-statistic |
|---|---|---|---|---|---|
| Positive correlation with RS during disapproval > anger | |||||
| dACC | 0 | 24 | 30 | .59 | 2.73 |
| 8 | 30 | 22 | .61 | 2.91 | |
| 14 | 24 | 40 | .50 | 2.18 | |
| 14 | 32 | 24 | .55 | 2.49 | |
| 6 | 38 | 36 | .54 | 2.43 | |
| 16 | 26 | 42 | .45 | 1.91 | |
| Positive correlation with RS during disapproval > disgust | |||||
| dACC | 0 | 24 | 30 | .66 | 3.26 |
| 8 | 30 | 22 | .62 | 2.95 | |
| 14 | 24 | 40 | .59 | 2.74 | |
| 14 | 32 | 24 | .48 | 2.04 | |
| 6 | 38 | 36 | .48 | 2.07 | |
| 16 | 26 | 42 | .59 | 2.71 | |
Note: All activations p < .05, uncorrected, 10-voxel extent threshold. RS = rejection sensitivity.
Negative correlations
When examining the neural regions that correlated negatively with rejection sensitivity during disapproval versus fixation, rejection sensitivity correlated negatively with activity in a single region, the subgenual ACC (subACC/VMPFC), (r = −.75, p < .005, 10-voxels; see Table 3 and Figure 5). Thus, individuals who scored higher in rejection sensitivity also exhibited less activity in subACC/VMPFC in response to the disapproval expressions. The fact that rejection-sensitive individuals showed reduced subACC/VMPFC activity to disapproving facial expressions fits with previous work demonstrating a role for the subACC/VMPFC in the extinction of conditioned fear responses in humans (Phelps, Delgado, Nearing, & LeDoux, 2004) as well as in signaling a less-threatening interpretation of a negative stimulus (Kim, Somerville, Johnstone, Alexander, & Whalen, 2003). Thus, it is possible that rejection-sensitive individuals may show reduced subACC/VMPFC activity to disapproving faces and thus are less able to regulate the negative responses to these stimuli.
Figure 5.
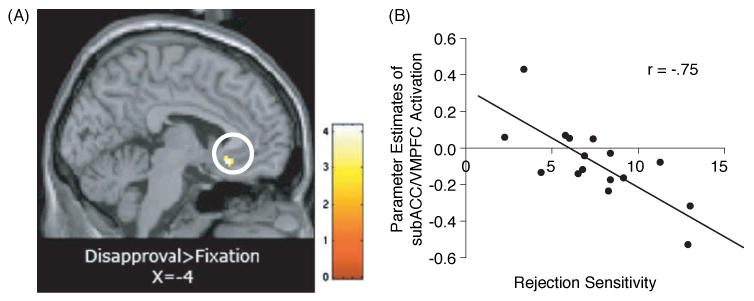
(A) SubACC/VMPFC activity (−4, 26, −12) in response to disapproval expressions compared to fixation that correlates negatively with self-reported rejection sensitivity scores (r = −.75; p < .005,10-voxels); (B) scatterplot showing the relationship between rejection sensitivity scores and subACC/VMPFC activity (−4, 26, −12) in response to disapproving expressions compared to fixation.
Somewhat unexpectedly, rejection sensitivity was also negatively correlated with activity in a variety of regions during the anger and disgust conditions relative to fixation (see Table 3). During anger versus fixation, higher levels of rejection sensitivity were associated with lower levels of activity in the dACC, subACC/VMPFC, left amygdala, and MPFC (p < .005, 10-voxels). During disgust versus fixation, higher levels of rejection sensitivity were associated with lower levels of activity in the right amygdala (p < .005, 10-voxels). This result can alternatively be interpreted as individuals high in rejection sensitivity showing more activity in these limbic and prefrontal regions during the rest (i.e., fixation) period, relative to viewing the anger or disgust expressions.
Functional connectivity during the disapproval condition relative to fixation
Connectivity analyses were completed to examine the relationship between dACC, which was hypothesized a priori to be moderated by rejection sensitivity, and other regions of the brain during the viewing of disapproving expressions compared to fixation. Parameter estimates were extracted from each of the regions of the dACC that correlated with rejection sensitivity and then entered as regressors into whole-brain random effects group analyses, comparing neural activity during disapproval to neural activity during fixation. There were no regions of activity that correlated with dACC at p < .005, 10-voxels; however, when reducing the extent threshold, results revealed that activity in the dACC (x = 14, y = 24, z = 40) was negatively correlated with activity in subACC/VMPFC (x = −4, y = 26, z = −12; r = −.63; p < .005, 6-voxels). Thus, individuals who showed greater activity in the dACC while viewing disapproving faces, compared to fixation, also showed a corresponding reduction in subACC/VMPFC activity. Interestingly, this is the precise region of subACC/VMPFC that was negatively correlated with rejection sensitivity.
Discussion
The purpose of the present study was to: (a) examine the neural signature associated with viewing disapproving facial expressions, which have not been investigated with neuroimaging methods, and (b) to examine how neural responses to disapproving faces are moderated by rejection sensitivity. To accomplish these goals, participants completed a measure of rejection sensitivity and were scanned while viewing disapproval, anger, and disgust facial expressions.
As expected, all three facial expressions (disapproval, anger, disgust) resulted in significant bilateral amygdala activity compared to fixation. This extends prior work showing amygdala activity in response to viewing negative emotional expressions, such as anger, fear, or disgust (Fitzgerald et al., 2006; Morris et al., 1996; Phillips et al., 1997; Whalen et al., 1998, 2001), and indicates that a disapproving facial expression may be interpreted as an inherently threatening emotional expression as well. Interestingly, when comparing neural activity to disapproving facial expressions versus anger expressions, there was significantly greater left amygdala activation, suggesting that disapproving faces may represent a more potent threat at the neural level than some other previously studied negative expressions. In addition, the finding of greater insula activity in response to disgust expressions relative to disapproval expressions provides additional evidence that distinguishes disapproval faces from other threatening facial expressions, highlighting the notion that disapproval may not just be a variant of disgust (cf. Ekman & Friesen, 1986).
Contrary to our predictions, however, individuals did not show greater dACC activity while viewing disapproving faces compared to fixation. In hindsight, this lack of a main effect for dACC may not be entirely surprising. The dACC is a region that has been shown to be involved in responding to personal threats to the self such as the distress associated with social exclusion (Eisenberger et al., 2003, 2007) or the distressing or unpleasant experience associated with physical pain (Rainville, Duncan, Price, Carrier, & Bushnell, 1997). In the present study, participants watched video clips of strangers demonstrating disapproving expressions, which may not have been experienced as personally threatening.
Nonetheless, as predicted, dACC activity was moderated by rejection sensitivity in response to viewing disapproving facial expressions. Specifically, individuals high in rejection sensitivity exhibited greater dACC activity in response to viewing the disapproving facial expressions than individuals low in rejection sensitivity. This was true even when disapproving facial expressions were compared directly with anger and disgust facial expressions. Thus, for individuals who are dispositionally sensitive to rejection, stimuli that signal social rejection (i.e., video clips of strangers demonstrating disapproving expressions) elicited activity in some of the same neural regions that are involved in the experience of social rejection. Because individuals high in rejection sensitivity are more likely to interpret ambiguous stimuli as rejecting (Downey & Feldman, 1996), these individuals may have interpreted these disapproving facial expressions as more personally threatening. It is also possible that individuals high in rejection sensitivity were more likely to become distressed by these disapproving facial expressions, either because they are more sensitive to these types of rejecting expressions or because these rejecting expressions are more likely to trigger memories of past episodes of rejecting experiences. Because we did not assess distress levels in response to viewing the disapproving faces, we cannot determine whether the greater dACC activity observed in rejection-sensitive individuals is related to greater experiences of distress or to a more sensitive detection of cues that predict social rejection. Future studies will be needed to disentangle these two alternatives.
Of particular interest is that the positive relationship between rejection sensitivity and dACC activity was not seen in response to the anger or disgust faces, suggesting that individuals sensitive to rejection exhibited a greater dACC response specifically to facial expressions indicating possible rejection, but not to threatening facial expressions in general. Although previous researchers have suggested that disgust expressions may actually represent social rejection (Amir et al., 2005; Rozin, Haidt, & McCauley, 1999) the results here qualify this claim. The selective dACC response to disapproving facial expressions by high rejection sensitive individuals is also consistent with previous work showing that high-rejection-sensitive individuals showed heightened autonomic reactivity in response to rejection-themed cues, but not to other negative (but non-rejection-themed) stimuli (Downey, Mougios, Ayduk, London, & Shoda, 2004).
Another interesting point is that rejection sensitivity correlated specifically with dACC activity to disapproving faces, but not with other limbic system activity (e.g., amygdala, insula), suggesting that dACC activity, rather than more general limbic system activity, is specifically responsive to these disapproving faces in high, relative to low, rejection-sensitive individuals. Again, this finding suggests that disapproving facial expressions may represent a distinct type of threat that has not been examined previously in fMRI studies and that the dACC may play a unique role in responding to this expression.
In light of these findings, disapproving face stimuli may be more appropriate to use than other face stimuli when assessing negative affective processes in clinical disorders that involve a heightened sensitivity to negative social experiences. For example, fear of rejection has been shown to be the primary cognition for individuals with social anxiety (Turner, Johnson, Beidel, Heiser, & Lydiard, 2003), and hypersensitivity to social threat cues is a key feature of depressed states (Allen & Badcock, 2003; Mathews, Ridgeway, & Williamson, 1996). Thus, a socially threatening facial expression that specifically conveys potential social rejection may be more effective in engaging the maladaptive affective processes present in these populations.
In addition to its role in personal threats to the self, dACC has also been theorized to play a role in conflict monitoring, in which the dACC monitors for conflicting response tendencies (Botvinick, Cohen, & Carter, 2004; Carter et al., 2000; MacDonald, Cohen, Stenger, & Carter, 2000). Indeed, some have suggested that the dACC activity observed in response to the Cyber-ball social exclusion paradigm (Eisenberger et al., 2003) is due to the fact that the exclusion episode is unexpected and thus conflicts with prior expectations to be included (Somerville, Heatherton, & Kelley, 2006). Based on these accounts of dACC function, it may be argued that the dACC activity seen in response to the disapproving facial expressions is the consequence of disapproving faces being more “unexpected” than anger or disgust faces. However, if this were the case, we would expect to see a main effect of greater dACC activity in response to the disapproving facial expressions compared to the anger or disgust faces. Instead, dACC activity in response to the disapproving facial expressions correlated with rejection sensitivity. Because rejection-sensitive individuals are presumably more likely to expect to see disapproving faces (Downey & Feldman, 1996), it is unlikely that the enhanced dACC activity observed in rejection-sensitive individuals is due to expectancy violations (cf. Somerville et al., 2006). Furthermore, rejection-sensitive individuals have previously been shown to have an emotional response to rejection-related stimuli (Downey et al., 2004). In that study, high-rejection-sensitive individuals exhibited amplified startle responses in response to rejection-themed images. As the startle response is a measure of autonomic nervous system activity, this finding supports the idea that rejection sensitive individuals' responses to rejection cues have an affective component and are not strictly reducible to cognitive expectation effects. Thus, rejection sensitivity is associated with emotional responses to rejection-related cues. It follows that neural responses that differ by level of rejection sensitivity while viewing rejection-related cues may be involved in this emotional process.
Rejection sensitivity also correlated negatively with subACC/VMPFC activity in response to the disapproving facial expressions. The fact that rejection-sensitive individuals showed reduced subACC/VMPFC activity to disapproving facial expressions is consistent with previous work demonstrating a role for the subACC/VMPFC in the extinction of conditioned fear responses in both animals and humans (Morgan, Romanski, & LeDoux, 1993; Phelps et al., 2004; Quirk, Russo, Barron, & Lebron, 2000). For example, conditioned fear responses, which normally decrease during extinction trials (when the conditioned stimulus is presented without the unconditioned stimulus), persist among rats with lesions to the VMPFC, suggesting that the VMPFC may be essential for inhibiting the conditioned response. Moreover, in humans, extinction of a conditioned fear response has been associated with reduced amygdala activation and increased subACC/VMPFC activation (Phelps et al., 2004). Thus, it is possible that decreased subACC/VMPFC activity to disapproving faces in rejection-sensitive individuals may reflect a diminished ability to regulate the negative responses to these disapproving facial expressions.4
Consistent with this idea, in a functional connectivity analysis, we found dACC activity to be negatively correlated with subACC/VMPFC activity. This result is also similar to previous findings showing an inverse relationship between subACC/VMPFC and amygdala activity when assessing the valence of certain stimuli (Kim et al., 2003). In that study, to the extent that surprised facial expressions were interpreted more negatively, participants showed reduced subACC/VMPFC activity and greater amygdala activity; conversely, to the extent that surprised facial expressions were interpreted more positively, participants showed greater subACC/VMPFC activity and reduced amygdala activity. Additionally, these two regions were negatively correlated with each other, suggesting that sub-ACC/VMPFC inputs to the amygdala may be involved in down-regulating the activity of the amygdala, leading to more positive interpretations of certain stimuli. In a similar manner, the present findings may suggest that individuals who interpret the disapproving facial expressions more negatively (i.e., those high in rejection sensitivity) show reduced subACC/VMPFC and greater dACC activity, whereas individuals who interpret the disapproving facial expressions less negatively (i.e., those low in rejection sensitivity) show greater subACC/VMPFC and reduced dACC activity. Consistent with these results, Somerville and colleagues (Somerville et al., 2006) recently observed that the receipt of gestures of social acceptance were associated with increased activity in the subACC/VMPFC.
Thus, it is possible that the reduced subACC/VMPFC activity observed in rejection-sensitive individuals in the present study may be indicative of biased appraisal processes, such that rejection-sensitive individuals are less able to interpret these disapproving facial expressions in a non-threatening way. The diminished ability to reinterpret these disapproving facial expressions as non-threatening may then be related to the increased dACC activity that was evidenced among those high in rejection sensitivity.
One possible confound in our study may have been the level of realism present in each of the facial expressions. Like the classic Ekman-style photographs of emotions, the anger and disgust expressions may have seemed exaggerated and thus less ecologically valid than the disapproving facial expressions. Future studies should involve more realistic dynamic facial expressions of anger and disgust in addition to disapproval.
Additionally, future studies should include a direct comparison of disapproval faces with contempt faces, in order to determine whether or not there are meaningful differences in the responses to these expressions at the neural level. Darwin's original description of the contempt expression focused on its role in displaying hatred for another person, specifically when that person is considered insignificant (Darwin, 1872/1998, p. 234). Accordingly, a contempt expression is dismissive, signaling a lack of interest in establishing or maintaining any sort of social relationship. In contrast, a disapproving face is more akin to the facial expression that a parent would direct at a child who is misbehaving, a signal, not of hatred, but of disappointment in the child's behavior. The disapproving facial expression acts as a signal or motivational cue to correct another's behavior and, perhaps, to improve the state of the social relationship. Thus, these facial expressions may trigger different types of neural activity that reflect being rejected with the inability to reconcile (contempt) versus the ability to make amends (disapproval).
In future studies, it would also be interesting to examine how neural responses to disapproving and other facial expressions would differ from the results presented here if participants were given more specific instructions for viewing the video clips, such as to “imagine the facial expressions are directed at you,” or “imagine what this person is thinking.” It is likely that participants in our study used a variety of cognitive processes while watching the video clips. Constraining what participants are thinking while viewing the video clips may provide stronger, more uniform neural responses to the expressions presented.
Finally, future studies should also include a “baseline” control condition other than a crosshair fixation to more tightly control for the viewing of facial stimuli. Future studies would also benefit by including a baseline condition other than “neutral” facial expressions, as these expressions have been shown to elicit amygdala activity (Fitzgerald et al., 2006) and can be interpreted as threatening (Somerville, Kim, Johnstone, Alexander, & Whalen, 2004).
In conclusion, the current study is the first to examine neural responses to disapproving facial expressions. In addition to finding significant bilateral amygdala activity to this threatening facial expression, we also found that individuals who scored higher in rejection sensitivity showed greater dACC and reduced subACC/VMPFC activity to these disapproving faces. Not only did individuals sensitive to rejection show a greater threat response to the facial expressions indicating possible rejection, but they also did so selectively, suggesting that different types of threatening facial expressions convey specific types of threats, with disapproving facial expressions primarily signifying a threat to social connection.
Over a hundred years ago, Darwin observed how different emotional facial expressions convey unique information, with each expression adapted for a specific purpose. Today, we are extending these observations to the neural level. Clearly, facial expressions other than those representing “basic emotions” can have a profound effect on our functioning and well-being. Further research in this area will enable us to better understand these effects. As Ovid observed centuries ago, “Often a silent face has voice and words” (Ars Amatoria, Bk. I, 574).
Acknowledgments
This research was supported by a National Institutes of Mental Health (NIMH) postdoctoral research fellowship (T32MH-019925) to NIE, a UCLA Health Psychology Program university graduate fellowship to LJB, a predoctoral research fellowship from NIMH to LJB as part of the UCLA Health Psychology Program (MH15750), and an NIMH grant to MDL (MH071521). We also appreciate the support provided by the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, the Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Northstar Fund, and National Center for Research Resources grants RR12169, RR13642 and RR08655.
Footnotes
Amygdala activity has been reported less consistently for disgust expressions than for either fear or anger expressions (Fitzgerald et al., 2006; Phillips et al., 1997; see Murphy, Nimmo-Smith, & Lawrence, 2003, for a meta-analysis).
Video clips were used in lieu of still photographs because the videos more accurately captured the facial expressions as they would be seen in real-life settings. Furthermore, dynamic expressions have been shown to improve recognition of the emotional content of facial expressions (Frijda, 1953; Wehrle, Kaiser, Schmidt, & Scherer, 2000) and have been associated with greater amygdala responses (Sato, Kochiyama, Yoshikawa, Naito, & Matsumura, 2004).
The neutral facial expression condition was originally included to serve as a comparison condition for the other facial expressions. However, because this condition produced greater limbic and prefrontal activity than the other facial expressions, a finding consistent with recent research (Fitzgerald et al., 2006), it was not used as a control condition in the current analyses. All results pertaining to the neutral condition will be presented in a separate report.
These results should be interpreted with caution as rejection sensitivity also correlated negatively with subACC/VMPFC while viewing anger faces relative to fixation.
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Lisa J. Burklund, University of California, Los Angeles, California, USA
Naomi I. Eisenberger, UCLA Cousins Center for Psychoneuroimmunology, Los Angeles, California, USA
Matthew D. Lieberman, University of California, Los Angeles, California, USA
References
- Adolphs R. Recognizing emotion from facial expressions: Psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Review. 2002;1:21–61. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Is the human amygdala specialized for processing social information? Annals of the New York Academy of Sciences. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. Journal of Cognitive Neuroscience. 2002;14(8):1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Allen NB, Badcock PBT. The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychological Bulletin. 2003;129:887–913. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. Journal of Personality and Social Psychology. 2005;88(4):589–604. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Biehl M, Matsumoto D, Ekman P, Hearn V, Heider K, Kudoh T, et al. Matsumoto and Ekman's Japanese and Caucasian Facial Expressions of Emotion (JACFEE): Reliability data and cross-national differences. Journal of Nonverbal Behavior. 1997;21(1):3–21. [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, et al. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Science. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: Common and differential networks. NeuroImage. 2006;31(2):906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. New York: Oxford University Press; 1998. Originally published 1872. [Google Scholar]
- Dickerson SD, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: Implications for emotional dysregulation. Biological Psychiatry. 2003;54(11):1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Downey G, Feldman SI. Implications for rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology. 1996;70(6):1327–1343. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Downey G, Mougios V, Ayduk O, London BE, Shoda Y. Rejection sensitivity and the defensive motivational system: Insights from the startle response to rejection cues. Psychological Science. 2004;15(10):668–673. doi: 10.1111/j.0956-7976.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams K. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding genetic risk for aggression: Clues from the brain's response to social exclusion. Biological Psychiatry. doi: 10.1016/j.biopsych.2006.08.007. in press. [DOI] [PubMed] [Google Scholar]
- Ekman P. Emotions revealed: Recognizing faces and feelings to improve communication and emotional life. New York: Times Books; 2003. [Google Scholar]
- Ekman P, Friesen WV. A new pan-cultural facial expression of emotion. Motivation and Emotion. 1986;10(2):159–168. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expression of facial affect. NeuroImage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frijda NH. The understanding of facial expressions of emotion. Acta Psychologica. 1953;9:294–362. [Google Scholar]
- Kesler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, et al. Neural substrates of facial emotion processing using fMRI. Brain Research. Cognitive Brain Research. 2001;11(2):213–226. doi: 10.1016/s0926-6410(00)00073-2. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. NeuroImage. 2003;18(1):156–168. doi: 10.1006/nimg.2002.1323. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mathews A, Ridgeway V, Williamson DA. Evidence for attention to threatening stimuli in depression. Behaviour Research and Therapy. 1996;34:695–705. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. The relationship among expressions, labels, and descriptions of contempt. Journal of Personality and Social Psychology. 2004;87(4):529–540. doi: 10.1037/0022-3514.87.4.529. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cognitive. Affective & Behavioral Neuroscience. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings Biological Sciences/The Royal Society. 1998;265(1408):1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrews C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–970. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rozin P, Haidt J, McCauley C. Individual differences in disgust sensitivity: Comparison and evaluations of paper-and-pencil versus behavioral measures. Journal of Research in Personality. 1999;33:330–351. [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Naito E, Matsumura M. Enhanced neural activity in response to dynamic facial expressions of emotion: An fMRI study. Cognitive Brain Research. 2004;20(1):81–91. doi: 10.1016/j.cogbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings Biological Sciences/The Royal Society. 1998;265(1409):1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Tanofsky-Kraff M, Wilfley DE, Salovey P. The Yale Interpersonal Stressor (YIPS): Affective, physiological, and behavioral responses to a novel interpersonal rejection paradigm. Annals of Behavioral Medicine. 2000;22(3):204–213. doi: 10.1007/BF02895115. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Categorization of facial expressions in children and adults: Establishing a larger stimulus set. Poster presented at the Cognitive Neuroscience Society annual meeting; San Francisco, CA. 2002. [Google Scholar]
- Turner SM, Johnson MR, Beidel DC, Heiser NA, Lydiard SB. The Social Thoughts and Beliefs Scale: A new inventory for assessing cognitions in social phobia. Psychological Assessment. 2003;15:384–391. doi: 10.1037/1040-3590.15.3.384. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wehrle T, Kaiser S, Schmidt S, Scherer KR. Studying the dynamics of emotional expression using synthesized facial muscle movements. Journal of Personality and Social Psychology. 2000;78(1):105–119. doi: 10.1037//0022-3514.78.1.105. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]


