Abstract
Patch–clamp recordings of CA1 interneurons and pyramidal cells were performed in hippocampal slices from kainate- or pilocarpine-treated rat models of temporal lobe epilepsy. We report that γ-aminobutyric acid (GABA)ergic inhibition in pyramidal neurons is still functional in temporal lobe epilepsy because: (i) the frequency of spontaneous GABAergic currents is similar to that of control and (ii) focal electrical stimulation of interneurons evokes a hyperpolarization that prevents the generation of action potentials. In paired recordings of interneurons and pyramidal cells, synchronous interictal activities were recorded. Furthermore, large network-driven GABAergic inhibitory postsynaptic currents were present in pyramidal cells during interictal discharges. The duration of these interictal discharges was increased by the GABA type A antagonist bicuculline. We conclude that GABAergic inhibition is still present and functional in these experimental models and that the principal defect of inhibition does not lie in a complete disconnection of GABAergic interneurons from their glutamatergic inputs.
A central issue in epilepsy research is the fate of inhibition. Disinhibition, i.e., a decreased inhibitory drive on principal neurons (1), is suggested to be the major factor responsible for epileptogenesis in human temporal lobe epilepsy (TLE) and animal models of chronic limbic seizures (2–6). However, a paradox has been reported because γ-aminobutyric acid (GABA)ergic inhibitory postsynaptic potentials are not seen underlying epileptiform discharges in principal neurons whereas they can be disclosed in the presence of glutamate antagonists (7–14). This led to the hypothesis that inhibition would not be present and/or functional during paroxysmal discharges (10, 15). The aim of the present study was to test whether spontaneous and evoked GABAergic inhibition that impinges on principal neurons in slices from control rats is still present and functional not only during paroxysmal discharges but also during steady–state (outside epileptiform discharges) in TLE. For this purpose, we have performed whole cell recordings from morphologically identified CA1 hippocampal interneurons and pyramidal neurons in hippocampal slices obtained from two animal models of TLE: kainic acid (KA)- or pilocarpine-treated rats. Most experiments were performed in physiological conditions, i.e., without glutamatergic receptor antagonists to examine the interactions between excitatory and inhibitory drives. We report that all of the various types of recorded interneurons, including basket cells, fire action potentials in response to stimulation of their glutamatergic synapses and provide efficient and functional GABAergic inhibition to pyramidal cells during spontaneous and paroxysmal activities. We also report that spontaneous and evoked paroxysmal discharges occur synchronously in pair recordings of interneurons and pyramidal cells in TLE. Our observations suggest that the inhibitory drive on CA1 pyramidal cells is recruited in TLE and that interneurons, notably those that innervate the soma of pyramidal cells, are readily activated by their glutamatergic inputs.
MATERIALS AND METHODS
KA and pilocarpine injections were performed on male Wistar rats (180 g) following established procedures (4, 16). Nineteen KA-treated and 14 pilocarpine-treated rats that had displayed spontaneous recurrent limbic seizures for at least 2 weeks were selected (1–12 months after drug injection). Animals that had no apparent seizures within the last 24 h before the slice experiment were selected. Fifteen age-matched rats were used for control experiments. Rats were perfused with modified artificial cerebrospinal fluid and 400 μm-thick slices were prepared with a Leica (Deerfield, IL) VT 1000E tissue slicer as previously described (14). Artificial cerebrospinal fluid contained (in mM) 124 NaCl, 3 KCl, 1.25 KH2PO4, 26 NaHCO3, 1.3 MgSO4·7H2O, 2 CaCl2, and 10 d-glucose and was aerated continuously with 95% O2 and 5% CO2. Slices from treated animals showing burst activity were selected using extracellular recordings following established procedures (17). Bipolar twisted NiCr wire stimulation electrodes (trimel insulated apart from 50 μm at the tip) were placed in the stratum radiatum of the CA1 area close to stratum pyramidale and 0.5 mm from the recording electrode. Spontaneous postsynaptic currents (PSC) and potentials (PSP) were recorded from CA1 pyramidal cells and interneurons with tight seal, whole cell patch–clamp pipettes. Microelectrodes had a resistance of 4–12 Mohm, and internal solutions of the following compositions were used (in mM): (i) for current clamp experiments: 135 K-gluconate, 2 MgCl2, 0.1 CaCl2, 1 EGTA, 2 Na2ATP, 10 Hepes, 0.5% biocytin (pH 7.25) and (ii) for voltage–clamp experiments, K-gluconate was replaced by Cs-gluconate. Osmolarity was between 265 and 275 mOsm. Access resistance was monitored throughout the experiments and was found to be constant. The temperature in the recording chamber was maintained at 30–32°C. Cells were kept at their resting membrane potential (in current clamp mode) and −60 mV or +20 mV (in voltage–clamp mode) for the analysis of glutamatergic or GABAergic spontaneous PSCs respectively. Biocytin ejection occurred passively, and the neurons were morphologically identified post hoc. 6-Cyano-7-nitro-quinoxaline-2,3-dione (CNQX) and d-2-amino-5-phosphonovaleric acid (D-APV) were a gift from Novartis (Bern, Switzerland). KA, bicuculline, pilocarpine, and biocytin were obtained from Sigma. CNQX, D-APV, and bicuculline were diluted in oxygenated artificial cerebrospinal fluid. Signals were fed to Axopatch 200A (Axon Instruments, Foster City, CA) amplifiers. All data were digitized (10 kHz) with a Labmaster (Axon Instruments) interface card to a personal computer and analyzed with the acquis 1 program (G. Sadoc, DIPSI, Asnières, France). Parameters were compared with a Student’s t test for paired samples. Because there was no statistical difference between the results obtained from pilocarpine- or KA-treated rats, data were pooled together. Values are expressed as means ± SEM.
To specifically address the functionality of perisomatic inhibition (18), surgical cuts were made in stratum radiatum and stratum oriens perpendicularly to the transverse axis, sparing stratum pyramidale. The stimulating electrode was located in stratum pyramidale on one side of the cuts (allowing the direct stimulation of axoaxonic and axosomatic cells) and the recording electrode on the other side. CNQX and D-APV were perfused in the bath to block glutamatergic neurotransmission.
After electrophysiological recordings, the slices remained in the chamber for 30–60 min to allow transport of biocytin throughout neuronal processes, and then they were fixed overnight at 4°C in a solution containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Slices were cryoprotected in sucrose and quickly frozen on dry ice, and 60 μm-thick sections were cut on a cryostat. Avidin-biotinylated peroxidase complex (Vector Laboratories) and 3,3′-diaminobenzidine tetrahydrochloride (Sigma) as chromogen were used to visualize filled cells. Some biocytin-injected neurons also were visualized with fluorescence in unsectioned slices that were processed for 24 h with avidin-d-fluorescein isothiocyanate (Vector Laboratories) diluted in saline phosphate buffer (0.1 M PBS, pH 7.4) containing 0.3% Triton X-100. Neurons were reconstructed using either a camera lucida device or the Neurolucida system (Microbrightfield, Colchester, VT). Interneurons were identified using morphological criteria (location of the soma, aspiny dendrites, and axonal arborization).
RESULTS
Morphological and Electrophysiological Properties.
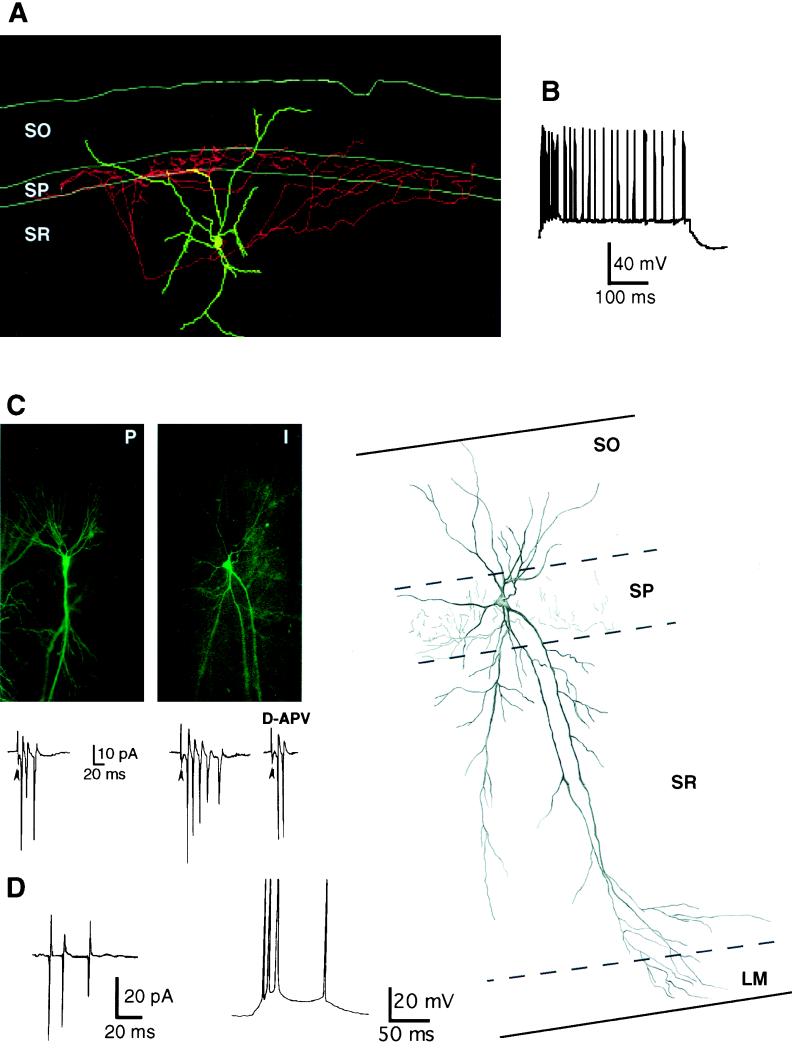
Somatic patch–clamp recordings from CA1 interneurons (n = 14) and pyramidal cells (n = 195) were performed in hippocampal slices from spontaneously epileptic rats 1–12 months after parenteral injection of pilocarpine or intracerebroventricular injection of KA. The 14 interneurons morphologically identified included: (i) two interneurons with a soma in the stratum oriens–alveus border, a horizontally oriented dendritic tree, and an axon that arborized in stratum oriens and stratum radiatum, (ii) three interneurons in stratum pyramidale including two perisomatic neurons (presumed basket or chandelier cells) with a pyramidal shape cell body, dendrites spanning all layers, and an axon arborization that was confined to stratum pyramidale (Fig. 1C); the third one exhibited a fusiform cell body and a bipolar dendritic tree with an apical dendrite reaching stratum lacunosum moleculare; its axonal arborization was mainly distributed to stratum oriens and stratum radiatum, (iii) seven interneurons had their cell body located in stratum radiatum: They included two presumed perisomatic interneurons with an ovoid cell body giving rise to several aspiny dendrites and a main axon terminal field in stratum pyramidale (Fig. 1A); the other five interneurons displayed an axonal arborization restricted to stratum radiatum, and (iv) two interneurons had their soma in stratum lacunosum moleculare and the axon collaterals in stratum radiatum. None of the 14 interneurons exhibited adaptation to depolarizing pulses (Fig. 1B); 9 of 14 interneurons fired spontaneously (outside spontaneous epileptiform discharges) in the cell attached configuration at a frequency of 0.07 ± 0.03 Hz. The firing consisted of single spikes, doublets, or triplets. The responses of these interneurons after the activation of their excitatory afferents are described at the end of Results.
Figure 1.
Interneurons are not disconnected from their excitatory afferents. (A) Neurolucida reconstruction of a biocytin-labeled interneuron from a KA-treated rat (3 months postinjection). This interneuron (presumed basket cell) with its soma located in CA1 stratum radiatum (SR) exhibited an extensive axonal arborization in the pyramidal cell layer (SP). (B) The same interneuron was characterized by a lack of adaptation on a depolarizing step. K-gluconate, resting membrane potential (RMP) −58 mV. (C) A pyramidal neuron (P) and an interneuron (I) located in CA1 were recorded simultaneously in a slice obtained from a KA-treated rat (2 months postinjection). The biocytin-filled cells were detected by fluorescence and are illustrated in the two panels. Stimulation in stratum radiatum evoked simultaneous epileptiform discharges in the pyramidal cell and the interneuron recorded in the cell attached configuration. Responses are shown below each frame. As in the following figures, arrowheads indicate stimulus artifact. D-APV treatment reduced the duration of the epileptiform discharge. On the right, the camera lucida reconstruction of the interneuron is illustrated. This interneuron (presumed chandelier or basket cell) displays a soma with a triangular shape located within stratum pyramidale (SP) and a smooth dendritic arborization that extends to all layers. The main axon originates from a proximal dendrite and ramifies exclusively throughout the pyramidal cell layer. (D) Spontaneous paroxysmal discharges recorded in the cell attached (Left) and current clamp mode (Right) in a stratum radiatum interneuron in a slice obtained from a pilocarpine-treated rat. K-gluconate, resting membrane potential (RMP) −61 mV. Note the apparent lack of IPSP underlying the burst in the current clamp mode.
Stimulation in stratum radiatum evoked typical graded epileptiform bursts in almost all of the pyramidal neurons (190 of 194). Spontaneous bursts were observed in 68 of 70 pyramidal neurons recorded in pilocarpine rats but in only 3 of 125 in kainate rats. Spontaneous and evoked epileptiform discharges were reduced after application of the N-methyl-d-aspartate receptor antagonist D-APV and were suppressed after treatment with both CNQX and D-APV (not shown), as we and others have reported (14).
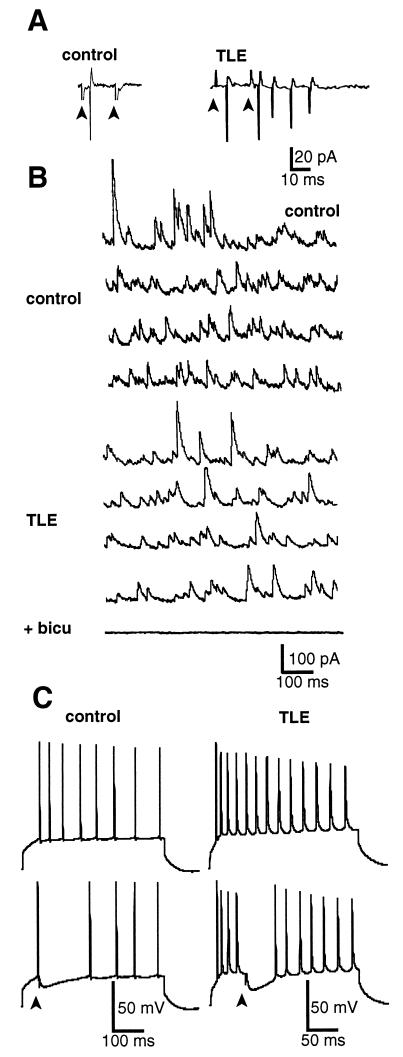
In keeping with earlier studies, paired-pulse inhibition was present in control but not in epileptic slices (8, 15, 17). This was readily shown using cell attached recordings (Fig. 2A) or the whole cell configuration (not shown). A large facilitation was observed in TLE (n = 195) when the two stimuli were applied at 20-ms intervals, in contrast to control cells (n = 15) in which the second spike was always abolished. Also in agreement with previous reports (7–14), inhibitory PSPs (IPSPs) were not seen during epileptiform discharges (n = 5; Fig. 1D but see ref. 14). However, in the presence of glutamate receptor antagonists (CNQX 10 μM plus APV 50 μM) to pharmacologically isolate GABAergic responses, a focal stimulation evoked an IPSP (Fig. 2C) or an inhibitory PSC (IPSC) (Fig. 3A).
Figure 2.
Pyramidal cells still receive inhibition despite the failure of paired pulse inhibition in TLE. (A) Pyramidal neurons were recorded in the cell attached configuration. (Left) Response of a pyramidal cell in control tissue to a paired pulse stimulation in stratum radiatum (interval 20 ms). Note the absence of a second discharge. (Right) Recording from a TLE pyramidal neuron. A large facilitation occurs after the second stimulus, indicating a failure of inhibition. (B) Spontaneous GABAergic activity is not modified in TLE. Spontaneous IPSCs were recorded from the somata of CA1 control (Upper) and TLE (Lower) pyramidal neurons. Continuous 4-s recordings of IPSCs are displayed. Neurons were voltage clamped at the reversal potential for glutamatergic currents, around +20 mV, Cs-gluconate. Bicuculline (10 μM) blocked all spontaneous activity. In this case, the time scale bar corresponds to 1 s (i.e., 10-s sweep). (C) Perisomatic inhibition blocked the generation of Na+ spikes in control [resting membrane potential (RMP) −64 mV] and TLE (RMP −67 mV) pyramidal cells (current clamp mode, K-gluconate). IPSPs were evoked by electrical stimulations applied in stratum pyramidale in the presence of glutamatergic antagonists (CNQX 10 μM and D-APV 50 μM).
Figure 3.
Pyramidal cells still receive functional inhibition during paroxysmal discharges in TLE. (A) A pyramidal neuron (KA model) was recorded in the whole cell configuration and voltage clamped (Cs-gluconate). Stimulation of stratum radiatum evoked a large glutamatergic current at the reversal potential of GABAA receptor-mediated currents (−60 mV) and a large GABAergic one at the reversal potential of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/N-methyl-d-aspartate receptor-mediated currents (0 mV). Biphasic currents were measured between these potentials. Application of CNQX (10 μM) and D-APV (50 μM) disclosed isolated IPSCs that reversed at −60 mV. Note the presence of spontaneous IPSCs. (B) Spontaneous paroxysmal discharges recorded (same protocol as in A) in a pyramidal cell of a pilocarpine rat. Note the large GABAergic current at +20 mV and the biphasic pattern at −40 mV. (C) Bicuculline treatment increased the duration of evoked epileptiform discharges in a KA pyramidal cell recorded in the cell attached configuration.
Functional GABAergic Inhibition in Pyramidal Cells.
The following observations suggest that GABAergic inhibition is functional in pyramidal neurons:
(i) GABAergic inhibition plays an important role during steady–state by providing a continuous background level of inhibition to the principal cells driving their membrane potential away from firing threshold. The frequencies of spontaneous IPSCs measured at the reversal potential of glutamatergic events (+20 mV) in control (16.8 ± 2.2 Hz, n = 10) and in TLE (17.3 ± 5.5 Hz, n = 22) were not statistically different (P = 0.9; Fig. 2B). These IPSCs were GABAergic because they were totally blocked after bicuculline application (Fig. 2B). Therefore, the ongoing GABAergic drive on pyramidal cells does not appear modified in TLE.
(ii) One of the functions of perisomatic inhibition is to control the firing of Na+ spikes in the soma of pyramidal neurons, as recently demonstrated by Miles et al. in the CA3 area (18). Using the same paradigm in CA1 pyramidal cells, we first verified, in control slices, that the pharmacologically and surgically isolated GABA type A (GABAA) receptor-mediated IPSP evoked by focal stimulation blocked the firing of Na+ spikes elicited by a depolarizing step (Fig. 2C; 3.2 ± 0.4 blocked spikes, n = 5). In TLE CA1 pyramidal cells, evoked GABAergic IPSPs were as efficient as in control (Fig. 2C; 3.0 ± 0.3 blocked spikes, n = 5, P = 0.7 between TLE and control).
Persistence of GABAergic Inhibition During Epileptiform Discharges.
The following observations suggest that GABAergic inhibition is present and functional during paroxysmal discharges in physiological conditions.
(i) GABAergic and glutamatergic currents were measured during evoked and spontaneous paroxysmal discharges. In whole cell recordings from pyramidal cells in the voltage–clamp mode, fast GABAergic events reversed between −55 and −65 mV, and glutamatergic events reversed between +10 and +20 mV. All neurons recorded from KA- and pilocarpine-treated rats (n = 195) were characterized by evoked polysynaptic excitatory PSCs (recorded at −60 mV) and large IPSCs (recorded at +20 mV). Biphasic currents were recorded between these two potentials (Fig. 3A). More direct evidence was obtained from the analysis of spontaneous interictal events recorded in pilocarpine-treated rats (Fig. 3B). Pyramidal cells (n = 68) showed a large inward current when recorded at −60 mV and a large outward current at +20 mV. These currents were respectively blocked by CNQX/D-APV (Fig. 3A) and bicuculline (not shown but see Fig. 2B), respectively.
(iii) The previous results suggest that GABAergic inhibition underlies epileptiform discharges at resting membrane potential. We then tested its efficacy in damping excitation. In all of the pyramidal cells tested (n = 15), perfusion of bicuculline increased the duration of both evoked and spontaneous epileptiform discharges (Fig. 3C). The number of action potentials was increased by 45 ± 14% after bicuculline treatment, suggesting that GABAergic inhibition was still active in pyramidal cells.
Glutamatergic Inputs to Interneurons.
Because GABAergic currents can be measured during glutamate-dependent epileptiform discharges and because bicuculline increases the duration of these discharges, inhibitory interneurons should fire action potentials via activation of their glutamatergic synapses during paroxysmal discharges. Indeed, stimulation in stratum radiatum evoked typical graded epileptiform bursts in 12 of the 14 recorded interneurons (Fig. 1C) and a single action potential in the other two. Spontaneous bursts were observed in four of five interneurons (Fig. 1D) recorded in pilocarpine rats. Spontaneous and evoked epileptiform discharges were reduced after application of the N-methyl-d-aspartate receptor antagonist D-APV (Fig. 1C) and were suppressed after treatment with both CNQX and D-APV (not shown). Finally, we have performed double patch–clamp recordings from four pairs of CA1 interneurons and pyramidal cells (not synaptically connected). Synchronized epileptiform discharges were evoked in three pairs (Fig. 1C); in the fourth one, the interneuron responded with a single action potential.
Thus, all recorded interneurons were active after the stimulation of their excitatory glutamatergic inputs during paroxysmal discharges in pyramidal cells.
DISCUSSION
The principal conclusion of the present study is that the various elements of the GABAergic inhibitory circuit are still operative in TLE. (i) There is a powerful spontaneous inhibitory drive that impinges on pyramidal cell somata because the frequency of on-going GABAergic currents is identical to that of controls. In control neurons, as much as 50% of this activity is network-driven, i.e., spike-dependent (19). This suggests that the firing of interneurons is partly responsible for the spontaneous GABAergic drive that impinges on CA1 pyramidal cells in TLE. (ii) Direct stimulation of GABAergic interneurons still prevents the generation of action potentials in pyramidal cells. (iii) Most importantly, in contrast to previous conclusions (15), the inhibitory circuit is also active during paroxysmal discharges in physiological conditions. Thus, paroxysmal activities were recorded in interneurons and were synchronous with those recorded simultaneously in pyramidal cells. (iv) The paroxysmal discharges of interneurons generate large IPSCs in pyramidal cells reflecting the functionality of the GABAergic synaptic inputs to pyramidal neurons. And (v) finally, these IPSCs are still efficient because bicuculline increases the duration of the discharges.
How can the presence of a functional inhibition be reconciled with the failure of paired pulse inhibition and the occurrence of spontaneous and evoked paroxysmal discharges? Two major, nonexclusive hypotheses have been proposed: (i) A major element that must be taken into account is the sprouting and the formation of novel glutamatergic synapses that would tip off the balance between inhibition and excitation in favor of the latter. Extensively described in the mossy fiber pathway (20–25), recent data indicate that sprouting also takes place in CA1 (26), supporting the hypothesis that an enhanced excitatory drive could facilitate the occurrence of epileptiform discharges. (ii) The “dormant basket cell” hypothesis suggests that disinhibition is due to a partial (15) or complete (10) disconnection of interneurons, in particular the basket cells, from their glutamatergic inputs. Therefore, interneurons would not be able to provide an efficient inhibitory drive to the pyramidal cells. The result at the origin of the hypothesis was that GABAergic interneurons did not degenerate in the dentate gyrus (2) or in the CA1 area (15). However, the use of more sensitive techniques recently demonstrated a clear loss of some populations of interneurons in both regions (4, 27). Our recordings from interneurons, including basket cells, exclude the complete disconnection theory (10) and cannot be readily reconciled with the dormant basket cell hypothesis, which suggests a “decreased direct activation of CA1 basket cells” (15). Finally, because the probable disconnection of interneurons from some of their excitatory afferents does not prevent their hyperexcitability (at least those we have recorded from), we suggest that this disconnection may not play a major role in the disinhibition of principal cells.
Other mechanisms affecting the strength of inhibition could also result in disinhibition of principal neurons: (i) a decrease of GABA release from GABAergic terminals and (ii) postsynaptic mechanisms such as a modification of the efficacy by Zn2+ (6) or Ca2+ (28) or the density of GABAA receptors. In fact, a recent study reported that the conductance of GABAA receptors is reduced by 50% in isolated CA1 hippocampal neurons in the pilocarpine model, providing the first direct evidence of a functional deficit of inhibition at the postsynaptic level (29). Such a deficit and the loss of interneurons projecting to the dendrites (27) could directly explain the occurrence of paroxysmal discharges in CA1 pyramidal cells if excitation is not sufficiently damped by inhibition anymore. It must be pointed out that the mechanisms resulting in disinhibition may be area-specific. Indeed, Gibbs et al. reported that, in contrast to CA1 pyramidal cells, the conductance of GABAA receptors was increased in dentate granule cells (29).
In conclusion, the remaining inhibition is still efficient and functional enough to keep CA1 pyramidal cells “under control” most of the time. However, the alterations affecting inhibition would lead to an unstable steady–state in which a slight perturbation would be sufficient to tilt the balance toward paroxysmal activities.
Acknowledgments
We thank Dr. G. L. Holmes for critical reading of the manuscript, D. Diabira for providing the lesioned animals, and Novartis (Bern, Switzerland) for the gift of CNQX and D-APV. This work was supported by Institut National de la Santé et de la Recherche Médicale and the Simone and Cino del Duca Foundation.
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- KA
kainic acid
- TLE
temporal lobe epilepsy
- PSP
postsynaptic potentials
- PSC
postsynaptic currents
- IPSP
inhibitory PSP
- IPSC
inhibitory PSC
- CNQX
6-cyano-7-nitro-quinoxaline-2,3-dione
- D-APV
d-2-amino-5-phosphonovaleric acid
- GABAA
GABA type A
References
- 1.Fisher R S. Brain Res Rev. 1989;14:245–278. doi: 10.1016/0165-0173(89)90003-9. [DOI] [PubMed] [Google Scholar]
- 2.Sloviter R S. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 3.Babb T, Pretorius J, Kupfer W, Crandall P. J Neurosci. 1989;9:2562–2574. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obenaus A, Esclapez M, Houser C R. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathern G W, Babb T L, Pretorius J K, Leite J P. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhl E, Otis T, Mody I. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- 7.Franck J, Kunkel D, Baskin D, Schwartzkroin P. J Neurosci. 1988;8:1991–2002. doi: 10.1523/JNEUROSCI.08-06-01991.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapur J, Stringer J, Lothman E. J Neurophysiol. 1989;61:417–426. doi: 10.1152/jn.1989.61.2.417. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima S, Franck J, Bilkey D, Schwartzkroin P. Hippocampus. 1991;1:67–78. doi: 10.1002/hipo.450010107. [DOI] [PubMed] [Google Scholar]
- 10.Bekenstein J, Lothman E. Science. 1993;259:97–100. doi: 10.1126/science.8093417. [DOI] [PubMed] [Google Scholar]
- 11.Williams S, Vachon P, Lacaille J-C. Neuroscience. 1993;52:541–554. doi: 10.1016/0306-4522(93)90404-4. [DOI] [PubMed] [Google Scholar]
- 12.Whittington M, Jefferys J. J Physiol (London) 1994;481:593–604. doi: 10.1113/jphysiol.1994.sp020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangan P, Rempe D, Lothman E. J Neurophysiol. 1995;74:829–840. doi: 10.1152/jn.1995.74.2.829. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch J C, Quesada O, Esclapez M, Gozlan H, Ben-Ari Y, Bernard C. J Neurophysiol. 1996;76:4185–4189. doi: 10.1152/jn.1996.76.6.4185. [DOI] [PubMed] [Google Scholar]
- 15.Sloviter R. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- 16.Ashwood T J, Wheal H V. Br J Pharmacol. 1987;91:815–822. doi: 10.1111/j.1476-5381.1987.tb11280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard C, Wheal H. J Physiol (London) 1996;495:127–142. doi: 10.1113/jphysiol.1996.sp021579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miles R, Toth K, Gulyas A I, Hajos N, Freund T F. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 19.Alger B E, Nicoll R A. Brain Res. 1980;200:195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
- 20.Tauck D, Nadler J. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Represa A, Tremblay E, Ben-Ari Y. Neuroscience. 1987;20:739–748. doi: 10.1016/0306-4522(87)90237-5. [DOI] [PubMed] [Google Scholar]
- 22.deLanerolle N C, Kim J H, Robbins R J, Spencer D D. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 23.Represa A, Robain O, Tremblay E, Ben-Ari Y. Neurosci Lett. 1989;99:351–355. doi: 10.1016/0304-3940(89)90472-2. [DOI] [PubMed] [Google Scholar]
- 24.Represa A, Ben-Ari Y. Trends Neurosci. 1992;92:69–78. [Google Scholar]
- 25.Sundstrom L E, Mitchell J, Wheal H V. Brain Res. 1993;609:321–326. doi: 10.1016/0006-8993(93)90890-y. [DOI] [PubMed] [Google Scholar]
- 26.Perez Y, Morin F, Jutras I, Beaulieu C, Lacaille J-C. Eur J Neurosci. 1996;8:736–748. doi: 10.1111/j.1460-9568.1996.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 27.Houser C R, Esclapez M. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 28.Staley K J, Soldo B L, Proctor W R. Nature (London) 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs J W, Shumate M D, Coulter D A. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]