Abstract
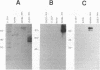
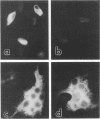
Mouse hepatitis virus-A59 (MHV-A59), a murine coronavirus, can utilize as a cellular receptor MHVR, a murine glycoprotein in the biliary glycoprotein (BGP) subfamily of the carcinoembryonic antigen (CEA) family in the immunoglobulin superfamily (G.S. Dveksler, M. N. Pensiero, C. B. Cardellichio, R. K. Williams, G.-S. Jiang, K. V. Holmes, and C. W. Dieffenbach, J. Virol. 65:6881-6891, 1991). Several different BGP isoforms are expressed in tissues of different mouse strains, and we have explored which of these glycoproteins can serve as functional receptors for MHV-A59. cDNA cloning, RNA-mediated polymerase chain reaction analysis, and Western immunoblotting with a monoclonal antibody, CC1, specific for the N-terminal domain of MHVR showed that the inbred mouse strains BALB/c, C3H, and C57BL/6 expressed transcripts and proteins of the MHVR isoform and/or its splice variants but not the mmCGM2 isoform. In contrast, adult SJL/J mice, which are resistant to infection with MHV-A59, express transcripts and proteins only of the mmCGM2-related isoforms, not MHVR. These data are compatible with the hypothesis that the MHVR and mmCGM2 glycoproteins may be encoded by different alleles of the same gene. We studied binding of anti-MHVR antibodies or MHV-A59 virions to proteins encoded by transcripts of MHVR and mmCGM2 and two splice variants of MHVR, one containing two immunoglobulin-like domains [MHVR(2d)] and the other with four domains as in MHVR but with a longer cytoplasmic domain [MHVR(4d)L]. We found that the three isoforms tested could serve as functional receptors for MHV-A59, although only isoforms that include the N-terminal domain of MHVR were recognized by monoclonal antibody CC1 in immunoblots or by MHV-A59 virions in virus overlay protein blot assays. Thus, in addition to MHVR, both the two-domain isoforms, mmCGM2 and MHVR(2d), and the MHVR(4d)L isoform served as functional virus receptors for MHV-A59. This is the first report of multiple related glycoprotein isoforms that can serve as functional receptors for a single enveloped virus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Baechi T., Haller O. Adult mouse hepatocytes in primary monolayer culture express genetic resistance to mouse hepatitis virus type 3. J Immunol. 1982 Sep;129(3):1275–1281. [PubMed] [Google Scholar]
- Aurivillius M., Hansen O. C., Lazrek M. B., Bock E., Obrink B. The cell adhesion molecule Cell-CAM 105 is an ecto-ATPase and a member of the immunoglobulin superfamily. FEBS Lett. 1990 May 21;264(2):267–269. doi: 10.1016/0014-5793(90)80264-j. [DOI] [PubMed] [Google Scholar]
- Barnett T. R., Kretschmer A., Austen D. A., Goebel S. J., Hart J. T., Elting J. J., Kamarck M. E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989 Feb;108(2):267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. S., Smith A. L. Mouse hepatitis virus nasoencephalopathy is dependent upon virus strain and host genotype. Arch Virol. 1986;91(3-4):247–256. doi: 10.1007/BF01314284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin N., Turbide C., Afar D., Bell J., Raymond M., Stanners C. P., Fuks A. A mouse analogue of the human carcinoembryonic antigen. Cancer Res. 1989 Apr 15;49(8):2017–2021. [PubMed] [Google Scholar]
- Bedinger P., Moriarty A., von Borstel R. C., 2nd, Donovan N. J., Steimer K. S., Littman D. R. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988 Jul 14;334(6178):162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton S. R., Stephensen C. B., Snyder S. W., Weismiller D. G., Holmes K. V. Coronavirus species specificity: murine coronavirus binds to a mouse-specific epitope on its carcinoembryonic antigen-related receptor glycoprotein. J Virol. 1992 Dec;66(12):7420–7428. doi: 10.1128/jvi.66.12.7420-7428.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler G. S., Pensiero M. N., Cardellichio C. B., Williams R. K., Jiang G. S., Holmes K. V., Dieffenbach C. W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991 Dec;65(12):6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Tunison L. A., Oldstone M. B. Host genetic control of mouse hepatitis virus type 4 (JHM strain) replication. I. Restriction of virus amplification and spread in macrophages from resistant mice. J Gen Virol. 1984 Sep;65(Pt 9):1543–1548. doi: 10.1099/0022-1317-65-9-1543. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989 Aug 25;264(24):14408–14414. [PubMed] [Google Scholar]
- Maddon P. J., McDougal J. S., Clapham P. R., Dalgleish A. G., Jamal S., Weiss R. A., Axel R. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988 Sep 9;54(6):865–874. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- McCuaig K., Turbide C., Beauchemin N. mmCGM1a: a mouse carcinoembryonic antigen gene family member, generated by alternative splicing, functions as an adhesion molecule. Cell Growth Differ. 1992 Mar;3(3):165–174. [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Pensiero M. N., Dveksler G. S., Cardellichio C. B., Jiang G. S., Elia P. E., Dieffenbach C. W., Holmes K. V. Binding of the coronavirus mouse hepatitis virus A59 to its receptor expressed from a recombinant vaccinia virus depends on posttranslational processing of the receptor glycoprotein. J Virol. 1992 Jul;66(7):4028–4039. doi: 10.1128/jvi.66.7.4028-4039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensiero M. N., Jennings G. B., Schmaljohn C. S., Hay J. Expression of the Hantaan virus M genome segment by using a vaccinia virus recombinant. J Virol. 1988 Mar;62(3):696–702. doi: 10.1128/jvi.62.3.696-702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M., Fuks A., Stanners C. P. Biliary glycoprotein, a member of the immunoglobulin supergene family, functions in vitro as a Ca2(+)-dependent intercellular adhesion molecule. Cell Growth Differ. 1990 Nov;1(11):527–533. [PubMed] [Google Scholar]
- Smith A. L., Cardellichio C. B., Winograd D. F., de Souza M. S., Barthold S. W., Holmes K. V. Monoclonal antibody to the receptor for murine coronavirus MHV-A59 inhibits viral replication in vivo. J Infect Dis. 1991 Apr;163(4):879–882. doi: 10.1093/infdis/163.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S., Click R. E., Plagemann P. G. Control of mouse hepatitis virus replication in macrophages by a recessive gene on chromosome 7. J Immunol. 1984 Jul;133(1):428–432. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Gaur A., Chan P. Y., Springer T. A. Internalization of a major group human rhinovirus does not require cytoplasmic or transmembrane domains of ICAM-1. J Immunol. 1992 May 15;148(10):3271–3274. [PubMed] [Google Scholar]
- Stohlman S. A., Frelinger J. A., Weiner L. P. Resistance to fatal central nervous system disease by mouse hepatitis virus, strain JHM. II. Adherent cell-mediated protection. J Immunol. 1980 Apr;124(4):1733–1739. [PubMed] [Google Scholar]
- Sturman L. S., Takemoto K. K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972 Oct;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Zimmermann W., Osthus-Bugat P., Schleussner C., Eades-Perner A. M., Barnert S., Von Kleist S., Willcocks T., Craig I., Tynan K. Long-range chromosomal mapping of the carcinoembryonic antigen (CEA) gene family cluster. Genomics. 1992 Apr;12(4):761–772. doi: 10.1016/0888-7543(92)90307-e. [DOI] [PubMed] [Google Scholar]
- Turbide C., Rojas M., Stanners C. P., Beauchemin N. A mouse carcinoembryonic antigen gene family member is a calcium-dependent cell adhesion molecule. J Biol Chem. 1991 Jan 5;266(1):309–315. [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Snyder S. W., Frana M. F., Holmes K. V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990 Aug;64(8):3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]