Abstract
Ribosomal RNA (rRNA) genes are down-regulated during osteogenesis, myogenesis, and adipogenesis, necessitating a mechanistic understanding of interrelationships between growth control and phenotype commitment. Here, we show that cell fate-determining factors [MyoD, myogenin (Mgn), Runx2, C/EBPβ] occupy rDNA loci and suppress rRNA expression during lineage progression, concomitant with decreased rRNA expression and reciprocal loss of occupancy by c-Myc, a proliferation-specific activator of rRNA transcription. We find interaction of phenotypic factors with the polymerase I activator upstream binding factor UBF-1 at interphase nucleoli, and this interaction is epigenetically retained on mitotic chromosomes at nucleolar organizing regions. Ectopic expression and RNA interference establish that MyoD, Mgn, Runx2, and C/EBPβ each functionally suppress rRNA genes and global protein synthesis. We conclude that epigenetic control of ribosomal biogenesis by lineage-specific differentiation factors is a general developmental mechanism for coordinate control of cell growth and phenotype.
Keywords: C/EBP, mitosis, MyoD, Runx
Ribosomal biogenesis is directly linked to cell growth and proliferation (1). Actively dividing cells require continuous ribosome synthesis to ensure that progeny cells have the capacity to support protein synthesis after cell division. A control point in the complex process of ribosome biogenesis is the transcriptional regulation of ribosomal RNA (rRNA) genes, which are transcribed by the RNA polymerase I (pol I) machinery. Although regulation of ribosomal biogenesis during the cell cycle is well documented (2), mechanisms that interrelate control of protein synthesis and lineage commitment are minimally understood.
Mesenchymal progenitor cells have the capacity to differentiate into cell lineages that include adipocytes, myoblasts, and osteoblasts (3). Phenotype determination requires integration of extracellular physiological cues and temporal upregulation of cell type-specific regulatory proteins. During differentiation, cells undergo a phase of rapid proliferation and then withdraw from the cell cycle, concomitant with the expression of lineage-specific transcription factors. For example, osteoblast differentiation of mesenchymal progenitors requires induction of Runx2 (4, 5), whereas adipogenesis is regulated by sequential upregulation of factors that include C/EBP (CCAAT/enhancer binding protein) -β, -δ, and -α and peroxisome proliferator-activated receptor (PPAR) γ (reviewed in refs. 6 and 7). Similarly, the basic helix–loop–helix (bHLH) transcription factors MyoD and Myogenin (Mgn) play essential roles for skeletal muscle lineage determination by directly regulating RNA pol II-mediated muscle-specific genes (8–10). Withdrawal of proliferating progenitors and execution of differentiation programs coincide with a significant decrease in rRNA synthesis (11, 12). One fundamental question is how RNA pol II-dependent phenotypic gene expression is coordinated with RNA pol I-mediated rRNA transcription during lineage commitment.
In this study, we demonstrate that during differentiation of mesenchymal progenitors into osteoblasts, myoblasts, or adipocytes, phenotypic regulatory factors critical for each lineage (e.g., Runx2, MyoD, Mgn, or C/EBP) suppress rRNA gene transcription and selectively interact with rRNA genes during interphase and mitosis. Our findings indicate that epigenetic regulation of rRNA synthesis by cell fate-determining factors is a broadly used mechanism for coordinating cell growth with lineage progression.
Results and Discussion
Cell Fate-Determining Factors Occupy rDNA Genes During Mesenchymal Cell Differentiation.
Phenotypic commitment in pluripotent cells is controlled by reciprocal expression of transcription factors that are specific for different lineages. To assess the expression of rRNA genes in distinct mesenchymal lineages, proliferating C2C12 cells were differentiated into either myotubes or osteoblasts and morphological changes were monitored by light microscopy [supporting information (SI) Fig. S1 A and C]. rRNA genes were down-regulated during differentiation into either myotubes (Fig. 1A) or osteoblasts (Fig. 1C). Each lineage exhibited induction of phenotypic transcription factors and differentiation markers, concomitant with down-regulation of genes for the other lineage. For example, sequential upregulation of the muscle regulatory factors MyoD and Mgn was observed in differentiated myoblasts, whereas the osteogenic factor Runx2 was down-regulated (Fig. 1B). Several muscle differentiation markers were also induced (Fig. S1B). Similarly, differentiated osteoblasts showed increased expression of Runx2 and osteoblast-related genes with a parallel decrease in MyoD as expected; Mgn, a late marker for muscle differentiation, is not present during osteoblast differentiation (Fig. 1D and Fig. S1D). We further investigated the expression of rRNA genes during mesenchymal cell differentiation into the adipocytic lineage. Consistent with our findings in muscle and bone differentiation, rRNA gene transcription and accumulation were reduced (Fig. 1E) with the expression of adipocyte markers (Fig. 1F). Together, these observations indicate that the induction of phenotypic regulatory proteins in each lineage coincides with the down-regulation of rRNA genes.
Fig. 1.
rRNA expression is down-regulated during mesenchymal cell differentiation. C2C12 cells were differentiated into either myoblast (A and B) or osteoblast (C and D) lineages, whereas 3T3-L1 cells were used for adipocyte differentiation (E and F). Cells were harvested at the indicated time points (i.e., days 0, 1, and 2). (A) Quantitative PCR analysis showed that both premature rRNA (○) and mature rRNA (●) levels were decreased during differentiation of all three lineages (A, C, and E). Western blot analysis confirmed the temporal expression of differentiation-related proteins specific for each lineage; MyoD and Mgn were upregulated during skeletal muscle differentiation (B), Runx2 expression increased during osteogenesis (D), and C/EBPα and β were induced during adipocyte differentiation (F). In contrast, expression of proliferation related proteins [e.g., Myc and cyclin A (CycA)] decreased as cells committed to various lineages, whereas levels of the rRNA transcriptional activator UBF remained unaltered. LaminB1 was used as a control for protein loading (B, D, and F).
Ribosomal DNA repeats contain functional Runx binding sites and E-boxes (the element recognized by bHLH proteins including Myc, MyoD, and Mgn) (13–15). Bioinformatics analysis reveals that C/EBP motifs are also present in the rRNA genes (Fig. 2A). We therefore postulated that phenotypic transcription factors are linked to rRNA transcription during cell fate determination. ChIP analysis showed that MyoD and Mgn sequentially occupy rDNA promoter regions (Fig. 2B, primer sets A and B) during skeletal muscle differentiation. MyoD exhibited maximal rDNA occupancy on day 1 at the rDNA region amplified by primer set B, whereas binding of Mgn was increased at day 2 (Fig. 2B) at the regions covered by both primer sets A and B. We also observe significant occupancy of rDNA (primer set A) by MyoD at day 0, which is consistent with detectable expression of MyoD in the premyoblast C2C12 cells (Fig. 1B). In contrast, increased rDNA occupancy by Runx2 was observed during osteogenic differentiation, with a parallel decrease in MyoD binding (Fig. 2C, primer sets A and B). Similarly, we found that C/EBPβ association with the rDNA regulatory regions increased during adipogenesis (Fig. 2D, primer sets A and C). Importantly, occupancy of rDNA by c-Myc, a known activator of rRNA transcription during proliferation (14, 15), decreased during lineage commitment (Fig. S2). Together, these findings indicate that phenotypic regulatory proteins have cell type-specific roles in down-regulation of rRNA gene transcription during lineage commitment.
Fig. 2.
Lineage-specific transcription factors occupy ribosomal DNA repeats in vivo. (A) Ribosomal DNA repeats contain binding sites for osteogenic Runx2 (▿), adipogenic C/EBP (●), and myogenic MyoD and Mgn (▴) transcription factors. Arrows indicate positions of ChIP primers used in this study. (B) ChIP assay using primer sets A and B showed sequential rDNA occupancy by MyoD (○) and Mgn (●) that is consistent with their temporal expression during myogenic differentiation of C2C12 cells. In contrast, the osteogenic Runx2 factor (◆) did not associate with rDNA repeats during muscle cell differentiation. No DNA was amplified from samples precipitated with IgG (◇). (C) When C2C12 cells were differentiated into osteoblasts by bone morphogenetic protein-2 treatment, the rDNA occupancy by Runx2 at regions A and B was increased, with a concomitant decrease in association of MyoD and Mgn with rDNA. (D) Binding of C/EBPβ (Δ) is increased at regions A and C of the rDNA locus during adipocyte differentiation of 3T3-L1 cells.
Phenotypic Transcription Factors Interact with UBF-1 During Lineage Progression.
To address mechanisms of rRNA control by phenotypic factors, we examined protein interactions with RNA pol I regulatory components during myogenesis as representative of mesenchymal cell differentiation. We initially assessed whether MyoD and Mgn localize with UBF-1, a key regulator of ribosomal gene transcription that is present in nucleoli during interphase and resides on acrocentric chromosomes at nuclear organizing regions (NORs) during mitosis (16, 17). Immunofluorescence (IF) microscopy revealed that an increasing subset (up to ≈55%) of UBF-1 foci colocalized with MyoD and Mgn at interphase nucleoli as myogenesis progressed (Fig. 3 A and B and Fig. S3). Consistent with our microscopy results, immunoprecipitation analysis showed that MyoD and UBF-1 interactions were increased during myotube formation (day 1) and sustained in multinucleated myotubes (day 2) (Fig. 3C). Similarly, Mgn and UBF-1 interactions become prominent during myogenic differentiation (day 2) (Fig. 3D). Analogous observations were made for Runx2 and UBF-1 during osteogenic induction of the same mesenchymal progenitors (data not shown). These findings further indicate that lineage-determining factors directly participate in RNA pol I-mediated transcriptional control of rRNA genes during differentiation. Importantly, MyoD was also present and colocalized with UBF-1 at NORs on mitotic chromosomes (Fig. 4). Hence, MyoD conveys intrinsic lineage specific regulatory cues through mitosis to progeny cells by an epigenetic mechanism.
Fig. 3.
Colocalization and interaction of MyoD and Mgn with UBF-1 increase during myogenesis. (A and B) (Left) Actively proliferating C2C12 cells, cultured on gelatin-coated coverslips, were differentiated into myotubes and analyzed for colocalization of MyoD and Mgn with UBF-1 at days 0, 1, and 2 by using IF microscopy. Percentages shown within the images indicate percent of UBF-1 foci that colocalize with MyoD or Mgn. (Right) Images reflect nine Z-sections of a representative nucleolus where MyoD or Mgn colocalize with UBF-1 (marked by yellow boxes). (A) MyoD and UBF-1 colocalization increased from day 0 to day 1 and persisted through day 2. Deconvolved images of interphase nuclei immunostained with MyoD (green) and UBF-1 (red) at various days of muscle differentiation are presented. (B) The colocalization between UBF-1 (red) and Mgn (green) is only observed at day 2 as Mgn is undetectable at days 0 and 1. (C and D) UBF-1 sequentially interacts with MyoD (C) and Mgn (D) during myoblast differentiation. MyoD and Mgn were immunoprecipitated at various days of muscle differentiation with specific antibodies or IgG as a control. Five percent of total cell lysates was used as input. Immunoprecipitates were resolved by SDS/PAGE followed by Western blot analysis. Membranes were then incubated with specific antibodies against UBF-1 or MyoD and Mgn.
Fig. 4.
MyoD colocalizes with UBF-1 at NORs during mitosis. Actively proliferating C2C12 cells, grown on gelatin-coated coverslips, were immunostained with MyoD (green) and UBF-1 (red), and counterstained with DAPI to visualize chromosomes. (Top) Cells were captured at different stages of mitosis. (Middle) Sections of representative NORs (shown by white boxes in Top) where MyoD localizes during mitosis are taken through the Z-plane. Numbers within each image indicate Z-sections from top (1) to bottom (9) of the cells. (Bottom) Colocalization of MyoD with UBF-1 is quantified by using the Line Scan function of the MetaMorph imaging software. Line graphs represent an average of colocalization between the two proteins through all Z-sections shown in Middle. The y axis shows pixel intensity of each signal, and the x axis represents length of the area scanned in pixels.
Lineage Commitment Factors Suppress rRNA Transcription and Global Protein Synthesis.
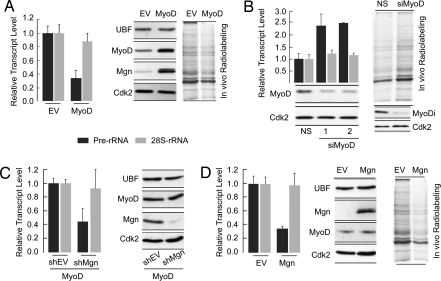
We directly addressed the functional relevance of lineage-specific factors in RNA pol I-mediated transcriptional control. Quantitative PCR analysis revealed that forced expression of MyoD suppressed rRNA gene transcription (≈2- to 2.5-fold), whereas mature 28 S rRNA levels remained essentially unchanged (Fig. 5A Left). As expected, elevation of MyoD upregulated muscle differentiation factors, including Mgn (Fig. 5A and Fig. S4A). In vivo radio-labeling of newly translated proteins revealed that MyoD expression decreased global protein synthesis, concomitant with a decrease in pre-rRNA transcripts (Fig. 5A Right). Consistent with MyoD-mediated suppression of rRNA genes, down-regulation of MyoD by RNA interference increased precursor rRNA levels and global protein synthesis (Fig. 5B), coincident with decrease levels of muscle differentiation markers (Fig. S4B). These findings establish MyoD as a lineage-specific transcriptional suppressor of rRNA genes in myoblasts.
Fig. 5.
Myogenic transcription factors MyoD and Mgn suppress rRNA gene transcription and global protein synthesis. (A) Actively proliferating C2C12 were infected with either vector backbone (EV) or MyoD retrovirus for 48 h. Cells were harvested for RT-PCR and Western blot analyses or were subjected to in vivo radio-labeling to assess global protein synthesis. For quantitative RT-PCR analysis, total cellular RNA was isolated from an equal number of cells to determine the effect of MyoD overexpression on rRNA transcription. Bar graphs represent rRNA levels normalized to EF-1α. Western blots show ectopic expression of MyoD. As expected, MyoD overexpression induced Mgn, whereas UBF-1 levels remained unaltered. Levels of Cdk2 are shown as control for protein loading. Cells were also metabolically labeled for 30 min with 35S-labeled amino acid mix to assess the effect of MyoD overexpression on global protein synthesis. (B) MyoD was down-regulated by two independent siRNA oligos (siMyoD 1 and 2). Each oligo reduced MyoD protein levels without affecting Cdk2, which was used a control for protein loading. Bar graphs show an increase in pre-rRNA transcript levels (normalized to EF-1 α), when MyoD was down-regulated by RNA interference. Mature 28S-rRNA remained unaltered. The autoradiograph shows increased global protein synthesis upon down-regulation of MyoD by a smart pool of four siRNA oligos against MyoD (Dharmacon). (C) Contribution of Mgn to the MyoD-mediated suppression of rRNA transcription was assessed by down-regulating MyoD-induced Mgn expression by shRNA. Cells infected with MyoD retrovirus were also transfected with either vector backbone (shEV) or shRNA against Mgn. Quantitative PCR analysis shows that MyoD-mediated suppression of rRNA transcription remained unaffected in the absence of Mgn. Down-regulation of Mgn was confirmed by Western blot analysis; shMgn had no effect on the protein levels of UBF-1, MyoD, or Cdk2. (D) Mgn was overexpressed in C2C12 cells by retroviral infection, and equal numbers of cells infected with vector backbone (EV) or Mgn retrovirus were analyzed for rRNA transcription, protein expression, or global protein synthesis. Bar graphs represent rRNA transcript levels normalized to EF1-α.
Because MyoD induces Mgn (Fig. 5A), which also binds E-boxes, we addressed whether Mgn also regulates rRNA genes. Using RNA interference against Mgn, we found that MyoD-mediated suppression of rRNA genes was maintained in the absence of Mgn (Fig. 5C and Fig. S4C). Forced expression of Mgn down-regulated rRNA expression and global protein synthesis (Fig. 5D and Fig. S4D). Thus, MyoD and Mgn can each independently suppress rRNA gene expression. Temporal expression of MyoD and Mgn (Fig. 1B) and sequential rDNA occupancy by both factors (Fig. 2B), together with these findings, suggest that MyoD and Mgn down-regulate rRNA gene transcription at different stages of skeletal muscle differentiation. Similarly, we observed down-regulation of RNA pol I-mediated control of rRNA gene expression when either the osteoblast-related factor Runx2 (Fig. 6A) or the adipocyte regulator C/EBPβ (Fig. 6B) were expressed. Taken together, our findings indicate that rRNA gene suppression is a shared property of phenotype-determining regulatory proteins.
Fig. 6.
Osteogenic Runx2 and adipogenic C/EBPβ transcription factors down-regulate rRNA transcription. (A) Actively proliferating C2C12 were infected with vector backbone (EV) or Runx2 adenovirus for 48 h. (B) Similarly, adipogenic 3T3-L1 cells were infected with vector backbone (EV) or C/EBPβ retrovirus. Bar graphs represent rRNA levels normalized with EF-1-α. Western blots show the expression of Runx2 (A) or C/EBPβ (B) and UBF-1 and Cdk2 (A and B).
In conclusion, this study establishes a common developmental event through which phenotypic regulatory factors coordinately control cell growth and lineage commitment. We find that suppression of ribosomal gene transcription and global protein synthesis during cell fate determination is mediated by interactions of lineage commitment factors with rRNA genes and cognate transcriptional regulators. It has been previously established that the cancer-related cell growth regulatory protein cMyc activates rRNA genes in proliferating cells (14, 15). During mesenchymal cell differentiation, we now show decreased occupancy of rDNA repeats by cMyc, concomitant with replacement by myogenic (MyoD and Mgn), osteogenic (Runx2), or adipogenic (C/EBP) regulatory factors that suppress ribogenesis. Therefore, we postulate that rRNA gene transcription is developmentally controlled by distinct mechanisms involving common cell growth regulatory activators and lineage-specific transcriptional suppressors. The adipogenic factor C/EBPβ has been shown to localize to mitotic chromosomes during the proliferative stage of 3T3-L1 adipocytes (18). In addition, we find that cell fate-determining factors including MyoD and Runx2 (13) associate with NORs during mitosis and transition into interphase nucleoli in progeny cells. Together, these findings provide a unique epigenetic mechanism for the establishment of phenotype-specific rRNA gene regulation and its maintenance during successive mitotic divisions of committed cells.
Materials and Methods
Cell Culture and Differentiation.
Mouse myoblast C2C12 cells were grown in DMEM containing 10% FBS growth medium. To differentiate the cells into myotubes, 90% confluent population of cells was switched to differentiation medium containing 2% horse serum. Cells were harvested at the time of switching cells from growth to differentiation medium (day 0) and after 24 h (day 1) and 48 h (day 2). For transdifferentiation of C2C12 cells into osteogenic lineage (4), cells were treated with BMP-2 (kindly provided by John Wozney, Wyeth Research, Cambridge, MA; 200 ng/ml) in DMEM containing 2% FBS. Cells were harvested at the time of BMP-2 treatment (day 0) and 24 h (day 1) and 48 h (day 2) after treatment. Differentiation of 3T3-L1 cells into adipocyte lineage has been described (19).
Plasmid Constructs.
MyoD and Mgn were expressed by using retroviruses containing cDNA for the respective gene. Both retroviruses have been reported (10, 20, 21). Retroviruses were produced by transfecting Bosc23 for 48 h, and C2C12 cells were infected with the medium containing the retroviruses. Mgn knockdown was obtained by shRNA-mediated silencing of the gene. The vector backbone and Mgn shRNA constructs have been published (22). C/EBPβ and Runx2 viruses used have been reported (19, 23).
In Situ IF Microscopy.
C2C12 cells, grown on gelatin-coated coverslips, were processed for in situ IF as described (13). In brief, cells were rinsed twice with ice-cold PBS and fixed in 3.7% formaldehyde in PBS for 10 min on ice. After rinsing once with PBS, cells were permeabilized in 0.1% Triton X-100 in PBS, and rinsed twice with PBSA (0.5% BSA in PBS) followed by 1-h incubation with primary antibody at 37°C. Rabbit polyclonal antibodies against MyoD (1:500; C-20) and Mgn (1:500; M-225) were used, and mouse monoclonal antibody was raised against UBF-1 (1:500; F-9). All antibodies were purchased from Santa Cruz Biotechnology. The secondary antibodies used were either anti-mouse Alexa 594 or anti-rabbit Alexa 488 (1:800; Molecular Probes). DNA was visualized by DAPI staining. Immunostaining of cell preparations was recorded with an epifluorescence Zeiss Axioplan 2 microscope equipped with a charged coupled device. Images were captured and analyzed by MetaMorph Imaging Software (Universal Imaging).
Coimmunoprecipitation and Western Blot Analysis.
Proliferating and differentiated C2C12 samples were washed with ice-cold PBS and harvested in sonication buffer [50 mM NaCl, 50 mM Tris (pH 8.0), 1% NP- 40, 25 mM MG132, and 1× protease inhibitor mixture (Roche)]. Lysates were incubated overnight at 4°C with 3 μg of rabbit antibodies against MyoD (C-20) or Mgn (M-225). Lysates were then incubated with protein A/G beads for 2 h, followed by four washes with wash buffer [50 mM NaCl, 20 mM Tris (pH 8.3), 0.5% Na-deoxycholate, 0.5% Nonidet P-40, 2 mM EDTA, 25 mM MG132, and 1× protease inhibitor mixture]. The total cell lysates and immunoprecipitated protein complexes were resolved by 8% SDS/PAGE and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore). Blots were incubated with different antibodies as follows: mouse MyoD mAb from BD PharMingen (1:1,000); Mgn (F5D; 1:500); rabbit polyclonal antibodies from Santa Cruz Biotechnology for MyoD (C-20; 1:1,000), C/EBPβ (C-19; 1:1,000), C/EBPα (14AA; 1:1,000), UBF-1 (F-9; 1:1,000), CyclinA (H- 432; 1:1,000), c-Myc (N- 262; 1:500), Cdk2 (M2; 1:1,000), Runx2 (M-70; 1:1,000), and LaminB1 (1:5,000; Zymed). Membranes were then incubated with HRP-conjugated secondary antibodies against rabbit or mouse (1:2,000). Proteins bands were visualized with a chemiluminescence detection kit (Perkin–Elmer Life Sciences).
ChIP.
ChIP assays were performed essentially as described (13). Briefly, cells were cross-linked in serum-free DMEM with 1% formaldehyde for 10 min, and the reaction was quenched by the addition of glycine at a final concentration of 250 mM for 10 min. Cells were washed twice with PBS and harvested in lysis buffer [150 mM NaCl, 50 mM Tris·HCl (pH 8.0), 1% Nonidet P-40, 25 mM MG-132, 1× complete protease inhibitor mixture]. After 10 min on ice, cells were sonicated to generate DNA fragments of ≈500–600 bp. Cell debris was precleared by centrifugation at 10,000 × g for 20 min. Five percent of the supernatant was kept as input. Rest of the supernatant containing protein–DNA complexes were incubated overnight with 4 μg of rabbit polyclonal antibodies directed against MyoD (C-20), Mgn (M-225), c-Myc (N-262), Runx2 (M-70), C/EBPβ (C-19), and UBF-1 (H-300) and 4 μg of normal rabbit IgG followed by 1.5-h incubation with protein A/G-conjugated agarose beads. Protein A/G bead complexes were washed with the following buffers: low salt [20 mM Tris·Cl (pH 8.1), 150 mM NaCl, 1% Triton X-100, 2 mM EDT, and 1× complete protease inhibitor mixture], high salt [20 mM Tris·Cl (pH 8.1), 500 mM NaCl, 1% Triton X-100, and 2 mM EDTA], LiCl [10 mM Tris·Cl (pH 8.1), 250 mM LiCl, 1% deoxycholate, 1% Nonidet P-40, and 1 mM EDTA], and twice in TE [10 mM Tris·Cl (pH 8.1) and 1 mM EDTA]. Protein–DNA complexes were eluted in 1% SDS/100 mM NaHCO3 and cross-linking was reversed by incubation at 65°C for 16 h in elution buffer and 300 mM sodium acetate (pH 5.2). DNA was extracted, purified, precipitated by phenol-chloroform procedure, and resuspended in TE for quantitative PCR. ChIP enrichment was determined as a quantitative measure reflecting the percentage of input. Primer set A is: forward, 5′-GCGGTTTTCTTTCATTGACC-3′, reverse, 5′-ACGACGCCTGGAACTCATAC-3′. Primer set B is: forward, 5′-TGTCAGGCGTTCTCGTCTC-3′, reverse 5′-GAGAGCACGACGTCACCAC-3′. Primer set C is: forward, 5′-GTCTCT CGGTCCCTTGTGAG-3′, reverse, 5′-ACGGGTCAGTCAGAGGAGAG-3′). They were designed in the promoter region of rDNA repeat. Nonspecific IgY enhancer was used as a negative control (forward, 5′-TGGTGGGGCTGGACAGAGTGTTTC-3′, reverse, 5′-GCCGATCAGAACCAGAACACC-3′).
RNA Isolation and Quantitative PCR Analysis.
Total RNA was isolated from C2C12 using TRIzol reagent (Invitrogen) and purified with a DNase-free RNA kit (Zymo Research). cDNA was generated from purified RNA by using a reverse-transcription reaction with random hexamer primers (Invitrogen) and subjected to quantitative PCR by using SYBR chemistry (Applied Biosystems). Pre-rRNA synthesis was assessed by using quantitative PCR with primers flanking early rRNA transcription processing sites. MRNA and rRNA transcript levels were normalized by internal control EF-1α (forward, 5′-GACCGTTCTTCCACCACTGATT-3′; reverse, 5′-AGCTTCTCTGACTACCCTCCACTT-3′). Other primers sequences used in this study were α-Αctin (forward, 5′-CAGAGCAAGCGAGGTATCC-3′; reverse, 5′-GTCCCCAGAATCCAACACG-3′), Desmin (forward, 5′-GTGGAGCGTGACAACCTGAT-3′; reverse, 5′-GATGGTCTCATACTGAGCCCG-3′), muscle creatine kinase (forward, 5′-GCGGAGGCAGTGTAACCCTTG-3′; reverse, 5′-CAGACCTCAGCAAGCACAACAATCAC-3′), alkaline phosphatase (AP) (forward, 5′-TTGTGCGAGAGAAAGAGAGAGA-3′; reverse, 5′-GTTTCAGGGCATTTTTCAAGGT-3′), osteocalcin (forward, 5′-CTGACAAAGCCTTCATGTCCAA-3′; reverse, 5′-GCGCCGGAGTCTGTTCACTA-3′), Runx2 (forward, 5′-CGACAGTCCCAACTTCCTGT-3′; reverse, 5′-CGGTAACCACAGTCCCATCT-3′).
Metabolic Labeling.
C2C12 cells were infected with retroviruses carrying either MyoD or Mgn cDNA or vector backbone (EV) for 48 h. Before harvesting, cells were treated with EasyTagTM Express [35S] protein labeling mix (200 mCu/ml) for 40 min (Perkin–Elmer). Cells were harvested in equal volume, and proteins were separated by SDS/PAGE (8%). Gel was dried and exposed to scientific imaging film (Kodak).
RNA Interference.
siRNA transfection for MyoD was performed according to standard techniques using Oligofactamine (Invitrogen). C2C12 cells grown at 30% confluence were transfected with two independent predesigned MyoD RNAi oligos purchased from Dharmacon. Cells were transfected with 50 nmol of each oligo for 48 h to down-regulate MyoD. For Mgn down-regulation, shRNA plasmid (22) was transfected with Fugene6 for 48 h.
Supplementary Material
Acknowledgments.
We thank Judy Rask for editorial assistance. These studies were supported by National Institutes of Health Grants CA082834, AR048818, and AR039588 (to G.S.S.), GM56244 and DK079239 (to A.N.I.), and AR049069 (to A.v.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800970105/DCSupplemental.
References
- 1.Hernandez-Verdun D, Roussel P. Regulators of nucleolar functions. Prog Cell Cycle Res. 2003;5:301–308. [PubMed] [Google Scholar]
- 2.Klein J, Grummt I. Cell cycle-dependent regulation of RNA polymerase I transcription: The nucleolar transcription factor UBF is inactive in mitosis and early G1. Proc Natl Acad Sci USA. 1999;96:6096–6101. doi: 10.1073/pnas.96.11.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigoriadis AE, Heersche JNM, Aubin JE. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: Effect of dexamethasone. J Cell Biol. 1988;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katagiri T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MH, et al. Transient up-regulation of CBFA1 in response to bone morphogenetic protein-2 and transforming growth factor β1 in C2C12 myogenic cells coincides with suppression of the myogenic phenotype but is not sufficient for osteoblast differentiation. J Cell Biochem. 1999;73:114–125. [PubMed] [Google Scholar]
- 6.Rangwala SM, Lazar MA. Transcriptional control of adipogenesis. Annu Rev Nutr. 2000;20:535–559. doi: 10.1146/annurev.nutr.20.1.535. [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 8.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 9.Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 10.Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 11.Bowman LH, Emerson CP., Jr Posttranscriptional regulation of ribosome accumulation during myoblast differentiation. Cell. 1977;10:587–596. doi: 10.1016/0092-8674(77)90091-5. [DOI] [PubMed] [Google Scholar]
- 12.Bowman LH. rDNA transcription and pre-rRNA processing during the differentiation of a mouse myoblast cell line. Dev Biol. 1987;119:152–163. doi: 10.1016/0012-1606(87)90217-x. [DOI] [PubMed] [Google Scholar]
- 13.Young DW, et al. Mitotic occupancy and lineage-specific transcriptional control of rRNA genes by Runx2. Nature. 2007;445:442–446. doi: 10.1038/nature05473. [DOI] [PubMed] [Google Scholar]
- 14.Arabi A, et al. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat Cell Biol. 2005;7:303–310. doi: 10.1038/ncb1225. [DOI] [PubMed] [Google Scholar]
- 15.Grandori C, et al. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat Cell Biol. 2005;7:311–318. doi: 10.1038/ncb1224. [DOI] [PubMed] [Google Scholar]
- 16.Roussel P, Andre C, Masson C, Geraud G, Hernandez-Verdun D. Localization of the RNA polymerase I transcription factor hUBF during the cell cycle. J Cell Sci. 1993;104:327–337. doi: 10.1242/jcs.104.2.327. [DOI] [PubMed] [Google Scholar]
- 17.Grummt I. Life on a planet of its own: Regulation of RNA polymerase I transcription in the nucleolus. Genes Dev. 2003;17:1691–1702. doi: 10.1101/gad.1098503R. [DOI] [PubMed] [Google Scholar]
- 18.Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salma N, Xiao H, Mueller E, Imbalzano AN. Temporal recruitment of transcription factors and SWI/SNF chromatin-remodeling enzymes during adipogenic induction of the peroxisome proliferator-activated receptor γ nuclear hormone receptor. Mol Cell Biol. 2004;24:4651–4663. doi: 10.1128/MCB.24.11.4651-4663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novitch BG, Mulligan GJ, Jacks T, Lassar AB. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy K, de lS I, Imbalzano AN. The myogenic basic helix–loop–helix family of transcription factors shows similar requirements for SWI/SNF chromatin remodeling enzymes during muscle differentiation in culture. J Biol Chem. 2002;277:33818–33824. doi: 10.1074/jbc.M205159200. [DOI] [PubMed] [Google Scholar]
- 22.Tang H, et al. CaM kinase II-dependent phosphorylation of myogenin contributes to activity-dependent suppression of nAChR gene expression in developing rat myotubes. Cell Signal. 2004;16:551–563. doi: 10.1016/j.cellsig.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Pratap J, et al. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25:8581–8591. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.