Abstract
Retinoid signaling plays a crucial role in patterning rhombomeres in the hindbrain and motor neurons in the spinal cord during development. A fundamentally interesting question is whether retinoids can pattern functional organization in the forebrain that generates a high order of cognitive behavior. The striatum contains a compartmental structure of striosome (or “patch”) and intervening matrix. How this highly complex mosaic design is patterned by the genetic programs during development remains elusive. We report a developmental mechanism by which retinoid receptor signaling controls compartmental formation in the striatum. We analyzed RARβ−/− mutant mice and found a selective loss of striosomal compartmentalization in the rostral mutant striatum. The loss of RARβ signaling in the mutant mice resulted in reduction of cyclin E2, a cell cycle protein regulating transition from G1 to S phase, and also reduction of the proneural gene Mash1, which led to defective neurogenesis of late-born striosomal cells. Importantly, during striatal neurogenesis, endogenous levels of retinoic acid were spatiotemporally regulated such that transduction of high levels of retinoic acid through RARβ selectively expanded the population of late-born striosomal progenitors, which evolved into a highly elaborate compartment in the rostral striatum. RARβ−/− mutant mice, which lacked such enlarged compartment, displayed complex alternations of dopamine agonist-induced stereotypic motor behavior, including exaggeration of head bobbing movement and reduction of rearing activity. RARβ signaling thus plays a crucial role in setting up striatal compartments that may engage in neural circuits of psychomotor control.
Keywords: basal ganglia, cell proliferation, retinoic acid, stereotypic behavior
A general principle of functional organization in the central nervous system is the compartmental arrangement of neuronal populations. The columnar organization of the cerebral cortex represents the most elaborate compartmental organization in the telencephalon, but compartmental organization also is present in the striatum. The striatum comprises two neurochemically distinct compartments, striosome (or patch) and the matrix (1–3). Unlike the columnar organization of the cerebral cortex, striosomes, which comprise ≈15–20% of the striatum, are embedded in the surrounding matrix to form a labyrinthine structure. Neurons in striosomes and in the matrix are generated during different time windows, differentiate at different rates into different neurochemical phenotypes, establish different connectivity with other brain regions, and degenerate at different rates in neurodegenerative diseases (2, 3).
Retinoid signaling, by virtue of its powerful patterning ability in developmental control, is involved in specification of body axis and building structural organs (4). Retinoid signaling plays a crucial role in patterning rhombomeres in the hindbrain and motor neurons in the spinal cord during development (5). A fundamentally interesting question is whether retinoids can pattern functional organization in the forebrain that generates cognitive behavior. Previous studies have shown that development of the intermediate part of telencephalon, which gives rise to the striatum in the mammalian telencephalon, is regulated by retinoid signaling (6–10). It is, however, unknown whether retinoid signaling is involved in control of compartmental formation during striatal development.
Retinoid signaling is transduced by binding to retinoic acid (RA) receptors (RARα, RARβ, RARγ) and retinoid X receptors (RXRα, RXRβ, RXRγ) that belong to the steroid/thyroid receptor superfamily (4). RARs and RXRs are ligand-activated transcription factors that can transactivate downstream target genes. Of the different subtypes of RARs, RARβ is preferentially expressed in the developing striatum (11–13). We report in the present study that RARβ signaling plays a critical role in setting up striatal compartmentation by differential regulation of the population sizes of striosomal cells along the rostrocaudal axis during development, and the enlarged striosomal compartment in the rostral striatum may modulate the neural circuits of psychomotor function.
Results
Aberrant Compartmentation in the Striatum of RARβ−/− Mutant Mice.
Striosomes express high levels of μ-opioid receptor (MOR1) and dynorphin but low levels of calbindin-D28K and met-enkephalin [Fig. 1 A and C; supporting information (SI) Fig. S1 A and C] (2, 3). MOR1 immunostaining showed that the areas of MOR1-positive striosomes were drastically reduced by 71.1% in RARβ−/− mutant striatum (Fig. 1 A, A′, and F). MOR1-positive striosomes disappeared mainly in the rostral striatum (Fig. 1 A, A′, and F) but were largely spared in the ventromedial striatum at middle and caudal levels (Fig. 1 B, B′, and F). Dynorphin was also reduced in the rostral mutant striatum (Fig. S1 A, A′, and F). Moreover, calbindin-poor zones (striosomes) were drastically diminished in the rostral striatum such that calbindin expression became largely homogeneous (Fig. 1 C′ and E′). The number of calbindin-positive neurons was, however, not significantly altered in mutant striatum (wild type, 1,315.5 ± 372.2/mm2; mutant, 1,297.0 ± 315.6/mm2, P = 0.887, n = 3). Immunostaining of met-enkephalin showed similar results to that of calbindin (Fig. S1 C, C′, E, and E′). Notably, in addition to the changes of striosomal markers, the striatal area was decreased in the rostral but not the caudal mutant striatum (Fig. 1G), suggesting a cytoarchitectural change. The perinatal striosomes express high levels of MOR1, dopamine- and cyclic adenosine 3′:5′-monophosphate-regulated phosphoprotein (DARPP-32), and tyrosine hydroxylase (TH). These three markers were also reduced primarily in the rostral part of newborn mutant striatum (Fig. S2 A–J′). Ebf-1, a marker for developing matrix (14), was increased in the rostral but not in the caudal part of newborn mutant striatum (Fig. S2 K–M). Defective striosomes also occurred prenatally at E16.5. At E16.5, DARPP-32-positive neurons were not yet organized into striosomal pattern in the wild-type striatum, but DARPP-32 neurons were significantly reduced in the mutant striatum (Fig. S3 B and B′), which indicated that the reduction of striosomes occurred before compartmental formation.
Fig. 1.
Aberrant compartments of striosomes and matrix in the adult striatum of RARβ−/− mutant mice. Immunostains of MOR1 (A, A′, B, B′) and calbindin (C, C′, D, D′) show that striosomes, as marked by MOR1-positive neuropil patches (A) and calbindin-poor zones (C), are reduced in the mutant striatum at rostral levels (A′, C′). The bracketed regions in C, C′ are shown at high magnification in E, E′. The areas of MOR1-positive patches are decreased in RARβ−/− mutant mice at the rostral level (F). The striosomal reduction of MOR1 and loss of calbindin-poor zones are less prominent in the mutant striatum at caudal levels (B, B′, D, D′, F). (G) The striatal area was reduced at the rostral but not the caudal level. (H–K) Loss of late-born S cells in RARβ−/− mutant striatum. The bracketed regions in H, H′ are shown at high magnification in I, I′, J, J′. Double immunostaining of MOR1 and BrdU (H–J′) shows that S cells pulse-labeled with BrdU at E12.75 and E13 (darkly stained black nuclei, arrowheads) are typically concentrated in MOR1-positive striosomes (brown neuropil patches, H, I) and the subcallosal lateral streak (H and J) in wild-type striatum. In contrast, only a few darkly stained BrdU-positive S cells (arrowheads) are present in the mutant striatum (H′, I′). BrdU-labeled cells are illustrated at high magnification in Insets (I, I′). Note that BrdU-positive S cells remain in the subcallosal lateral streak of mutant striatum (J′). (K) The number of BrdU-positive S cells is decreased in the striatal proper of mutant striatum but not in the lateral streak. **, P < 0.01, ***, P < 0.001, Student's t test, n = 3. (Scale bars in A for A, A′, 500 μm; in B for B, B′, 500 μm; in C for C, C′, 500 μm; in D for D, D′, 500 μm; in E for E, E′, 200 μm; In H′ for H, H′ 500 μm; in I′ for I–J′, 100 μm.)
Loss of Late-Born Striosomal Neurons in RARβ−/− Mutant Mice.
The concurrent decreases of several striosomal markers suggested that RARβ-null mutation might affect neurogenesis and/or survival of striosomal neurons. Striosomal cells (S cells) and matrix cells (M cells) finish their last mitosis at different time windows (15, 16). The majority of S and M cells can be pulse-labeled with BrdU at E11.5–E13.5 and after E16.5, respectively, in the mouse. Within the S cell population, most S cells in the caudal striatum and the subcallosal lateral streak are born at E11–E12.5, whereas most S cells in the rostral striatum are born later at E12.5–E13.5 in the mouse (17) (W.-L.L. and F.-C.L., unpublished observations).
Because RARβ mutation-induced decreases of S markers primarily occurred in the rostral striatum, we pulse-labeled rostral S cells with two injections of BrdU at E12.75 and E13. Clusters of BrdU-labeled S cells were drastically reduced by 88.2% in the rostral RARβ−/− mutant striatum (Fig. 1 H, H′, I, I′, and K). Similar findings were observed in the newborn mutant striatum (Fig. S3 A, A′, C, and C′). The subcallosal lateral streak, which comprised early-born S cells, remained largely unaffected in the rostral mutant striatum (Fig. 1 J, J′, and K). Importantly, the loss of late-born S cells occurred at E12.75 before the segregation of S and M cells into compartments, because the reduction of BrdU-labeled cells was detected in the mutant lateral ganglionic eminence (LGE, striatal anlage) at 28 h postinjection of BrdU when the BrdU-labeled postmitotic cells had migrated into the differentiated mantle zone but were not yet organized into compartments (Fig. 2A, A′, and C).
Fig. 2.
Defective neurogenesis of late-born S cells in striatal anlage of RARβ−/− mutant embryos. (A, A′, C) Twenty-eight hours after a single pulse labeling of BrdU at E12.75, more BrdU-positive S cells (green) migrate into the Ki67-negative differentiated mantle zone (MZ) of wild-type LGE (A) than that in the mutant LGE (A′, C). (B, B′, D, E) The number of S cells pulse-labeled with BrdU for 1 h at E12.75 is reduced in the VZ of LGE but not in the VP (E). The arrowheads in B, B′ indicate the boundary between the LGE and the VP. The reduction of cell proliferation also is temporally specific, because it occurs only in the S progenitor pulse-labeled with BrdU at E12.75 (late-born S cells) but not at E11.5 (early-born S cells; D), nor does it occur in the progenitors of matrix (M) cells pulse-labeled with BrdU at E16.5 (D). (F–I) Reduction of neurogenesis markers in RARβ−/− mutant LGE. Ccne2 mRNA is decreased in the mutant E12.75 LGE and VP (F, F′, H). The VP and the LGE are indicated by the regions between the double and single arrowheads and between the single arrowhead and arrow, respectively, in F, F′. Mash1 mRNA is reduced in E13.5 RARβ−/− mutant LGE (G, G′, I). *, P < 0.05; ***, P < 0.001, Student's t test. All experiments were repeated at least three times. CTX, cortex; Sep, septum; SVZ, subventricular zone; VP, ventral pallium. (Scale bars in A for A, A′; in B for B, B′; in F for F, F′; and in G for G, G′, 100 μm.)
Defective Neurogenesis of Late-Born Striosomal Cells in Striatal Anlage of RARβ−/− Mutant Embryos.
The loss of late-born S cells in the mutant striatum might be due to aberrant cell death and/or defective neurogenesis induced by RARβ-null mutation. We assayed cell death by TUNEL staining. The number of apoptotic cells in the wild-type LGE did not significantly differ from that in the mutant LGE at E12.75, E13.5, E14.5, and E15.5 (Fig. S4), which ruled out cell death as the primary cause of cell loss.
In addition to the mantle zone, low levels of RARβ were expressed in the ventricular zone (VZ) of LGE as detected by RT-PCR and in situ hybridization (Fig. S5), which is consistent with the report that RARβ is expressed by proliferating progenitors in neurospheres derived from striatal anlage (18). To determine whether the reduction of S cells was due to defective proliferation of S progenitors in the germinal zones, we pulse-labeled late-born S progenitors with BrdU at E12.75 and examined the BrdU incorporation rate at 1 h after BrdU injection. BrdU-positive cells were reduced by 16.7% in the VZ of RARβ−/− mutant LGE (Fig. 2 B and B′, D, and E). The reduction was specific to the LGE, because it did not occur in the ventral pallium of cortical anlage (Fig. 2E). In parallel to the defective proliferation, cyclin E2 (Ccne2), a cell cycle protein regulating transition from G1 to S phase (19), was decreased by 16.1% in the mutant LGE (Fig. 2 F, F′, and H). Moreover, the proneural gene Mash1 was also reduced by 19.3% in the mutant LGE (Fig. 2 G, G′, and I). BrdU-positive cells tended to decrease in the mutant subventricular zone (SVZ), a secondary proliferative population in the germinal zones (86.2 ± 7.5% of wild type, P = 0.0718, n = 6).
The deficit in cell proliferation was specific to late-born S cells, because pulse labeling of early-born S cells at E11.5 or M cells at E16.5 with BrdU did not show significant differences of BrdU-positive cells in the LGE/developing striatum between the wild-type and RARβ−/− mutant embryos (Fig. 2D). These results are in good accordance with the aforementioned findings that early-born S cells and M cells were spared in the adult mutant striatum (Fig. 1 F and K). Note that the loss of late-born S progenitors was not due to depletion of precursor pools by precocious differentiation, because no apparent increase of TuJ1-positive neurons was observed in E12.75 and E13.5 mutant LGE (data not shown).
RA Increased Cell Proliferation of Striatal Progenitors.
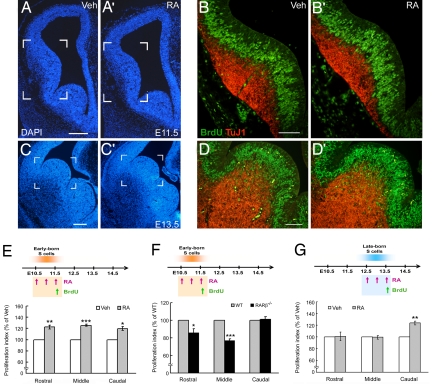
It remained a puzzle within the S cell population why RARβ mutation primarily targeted the late-born S cells, whereas it relatively spared the early-born S cells. A possible account for this differential effect is that the early-born S cells might be incompetent to transduce RA signals. To test this hypothesis, wild-type mouse embryos were maternally treated with all-trans RA (5 mg/kg) every 12 h from E10.5 to E11.5 and then pulse-labeled with BrdU for 1 h before embryo culling. The RA treatments resulted in increases of BrdU-positive cells in the VZ of LGE at rostral, middle, and caudal levels (Fig. 3B, B′, and E) and decreases of TuJ1-positive areas in the mantle zone (Fig. 3 B and B′). These results indicated that early-born S progenitors were in fact competent to transduce RA signals by expanding its population.
Fig. 3.
RA-induced increases of cell proliferation in striatal anlage. Embryos were maternally treated with all-trans RA (5 mg/kg) every 12 h from E10.5 to E11.5 (A, A′, B, B′, E, F) or E12.5 to E13.5 (C, C′, D, D′, G) and were then pulse-labeled with BrdU for 1 h before culling. RA treatments during E10.5–E11.5 result in increases of BrdU-positive cells (green nuclei) in the rostral, middle, and caudal levels of E11.5 wild-type LGE (B, B′, E), but not in RARβ−/− mutant LGE at the rostral and middle levels (F). The RA treatments during E12.5–E13.5 resulted in increases of BrdU-positive cells in the caudal (D, D′, G) but not the rostral and middle LGE (G). The bracketed regions in A, A′, C, C′ are shown at high magnification in B, B′, D, D′, respectively. *, P < 0.05; **, P < 0.01, ***, P < 0.001, Student's t test. All experiments were repeated at least three times. (Scale bars in A for A, A′, 200 μm; in B for B, B′, 100 μm; in C for C, C′, 200 μm; and in D for D, D′, 100 μm.)
To further test whether the RA-enhanced cell proliferation was mediated through RARβ signaling, the same set of experiments were performed in RARβ−/− mutant embryos and their wild-type littermates. RA treatments did not effectively increase proliferation of early-born S cells in RARβ−/− mutant LGE at rostral and middle levels (Fig. 3F), which indicated that the lack of RARβ largely prevented RA from increasing proliferation of early-born S cells.
We also tested whether exogenous RA could alter proliferation of late-born S cells by maternally treating wild-type mouse embryos with RA every 12 h from E12.5 to E13.5 and then pulse-labeling with BrdU for 1 h before culling. The RA treatments did not alter cell proliferation in the rostral and middle parts of E13.5 LGE (Fig. 3G). We surmised that this might be due to high levels of endogenous RA present in the rostral/middle LGE at E12.5–E13.5, which could limit the effect of exogenous RA (see below, Fig. 4B, E, and H). Accordingly, RA induced-cell proliferation should occur in the region where endogenous RA was low. Indeed, exogenous RA increased BrdU-positive cells in the VZ of caudal part of E13.5 LGE (Fig. 3 D, D′, and G), where a low level of endogenous RA was present (see below, Fig. 4 C, F, and I).
Fig. 4.
Spatiotemporal regulation of endogenous RA in the LGE. None or at most few Raldh3-positive cells are present in E11.5 LGE (A). By E13.5, many Raldh3-positive cells are present in the rostral/middle part of LGE (B), but few Raldh3-positive cells are present in the caudal LGE (C). (D–I) Detection of endogenous RA in the LGE with the RA reporter cells assay. Few X-gal-positive Sil-15 reporter cells are present in the coculture of E11.5 LGE (D and G) or the caudal part of E13.5 LGE (F and I), whereas many X-gal-positive reporter cells are present in the coculture of rostral/middle part of E13.5 LGE (E and H). The bracketed regions in D–F are shown at high magnification in G–I, respectively. (Scale bars, in A–C, 200 μm; in D for D, E, 500 μm; in F, 500 μm; and in G for G–I, 50 μm.)
Chronic RA Treatments Resulted in Enlarged Caudal Striosomes.
We further assayed the effects of chronic RA treatments during the entire neurogenesis of S and M cells. The embryos treated with RA every 12 h from E11.5 to E17.5 had enlarged DARPP-32- and GluR1-positive striosomes in the caudal striatum (Fig. S6). No significant effect was observed in rostral striosomes, which contained late-born S cells, nor did the population of M cells appear to be affected by the RA treatments (data not shown). Because striosomes in the caudal striatum were made up of early-born S cells, these results were consistent with the findings that exogenous RA mainly increased proliferation of early-born S cells (Fig. 3 B, B′, and E) and further suggest that as a consequence, more early-born S cells are recruited to caudal striosomes.
RA Directly Regulated Proliferation of Striatal Progenitor-Derived Cells in Vitro.
To further determine whether RA could directly regulate proliferation of striatal progenitors, we used the ST14A cell line, which was immortalized from rat striatal progenitors during the neurogenesis time window of S cells at E14 (20). The proliferating ST14A cells expressed RARβ and RXRβ transcripts (Fig. S7D). All-trans RA (1 μM) increased proliferation of ST14A cells, because BrdU-labeled cells were significantly increased by 142.3% with RA treatment (Fig. S7 B and C). RA also increased Ccne2 and decreased the differentiation marker of microtubule-associated protein 2 (Fig. S7D).
Spatiotemporal Regulation of Endogenous Levels of RA in Striatal Anlage.
Given that early-born S cells are capable of responding to RA signals, why is this cell population less affected by RARβ mutation? We postulated that the concentration of RA might be very low in E10.5–E11.5 LGE when the early-born S cells undergo neurogenesis. During early telencephalic development, a major RA source for the LGE is synthesized by retinaldehyde dehydrogenase 3 (Raldh3) (10, 21), although Raldh1 is present in the mesostriatal afferents (22). We found that few cells expressing Raldh3 mRNA were present in E11.5 LGE (Fig. 4A). In contrast, when the late-born S cells undergo neurogenesis at E12.5–E13.5, many Raldh3-positive cells were present in the rostral/middle LGE but not in the caudal LGE (Fig. 4 B and C) (21). We further determined the endogenous RA level in the LGE with a coculture assay. Using the Sil-15 RA reporter cells, RA produced by explant tissue could be detected by activation of the β-galactosidase reporter gene (9, 23). Few X-gal-positive cells were found when cocultured with E11.5 LGE (Fig. 4 D and G). In contrast, many X-gal-positive cells were detected when cocultured with the rostral (Fig. 4 E and H) but not the caudal part of E13.5 LGE (Fig. 4 F and I). These results confirmed that endogenous RA in the LGE was very low during the neurogenesis time window of early-born S cells, whereas a substantial level of RA was present during the neurogenesis time window of late-born S cells in the rostral LGE.
Alterations of Dopamine Agonist-Induced Stereotypic Motor Behaviors in RARβ−/− Mutant Mice.
The selective loss of rostral striosomes in RARβ−/− mutant mice provided a unique opportunity to look into the functional significance of S compartments at the behavioral level. We treated the mice with apomorphine, a stereotypy-eliciting dopamine agonist, and examined the stereotypic motor behaviors, including head bobbing, rearing, and grooming. With the vehicle treatment, wild-type and mutant mice had similar activities of locomotion, head movement, rearing, and grooming (Fig. 5 A–C; data not shown). Apomorphine (3 mg/kg) increased the locomotor activity in wild-type and RARβ−/− mutant mice to similar degrees (Fig. 5A). Apomorphine at the dosage of 3 mg/kg was ineffective in inducing repeated head bobbing in wild-type mice, but it drastically induced stereotypic head-bobbing movement in RARβ−/− mutant mice at 20 and 50 min after injection (P < 0.001, two-way ANOVA; Fig. 5B), which suggested the head-bobbing behavior was exaggerated in RARβ−/− mutant mice. Apomorphine at 3 mg/kg was also ineffective in altering the rearing activity in wild-type mice (Fig. 5C), but the rearing activity was completely lost in RARβ−/− mutant mice at 20 min after apomorphine injection (P < 0.001, two-way ANOVA; Fig. 5C). The grooming activity tended to decrease in apomorphine-treated RARβ−/− mutant mice, but the decrease did not reach statistical significance (data not shown).
Fig. 5.
Behavioral analyses of RARβ−/− mutant mice. (A) The locomotor activity of RARβ−/− mutant mice does not differ from wild-type mice either with the challenges of vehicle or apomorphine (Apo, 3 mg/kg) at 20 and 50 min after drug injections. (B) Apomorphine significantly increases the head-bobbing movement in RARβ−/− mutant mice at 20 and 50 min after the drug injection compared with wild-type mice. Note that apomorphine at this low dose does not increase head bobbing in wild-type mice. (C) Apomorphine (3 mg/kg) completely inhibited the rearing activity of RARβ−/− mutant mice at 20 min after injection. ***, P < 0.001, two-way ANOVA, Bonferroni's post hoc test. Apo-20, Apo-50, Veh-20, Veh-50: 20 and 50 min after the injections of apomorphine or its vehicle.
Discussion
Our study provides previously undescribed genetic evidence that retinoid receptor signaling plays a crucial role in patterning the functional organization in the striatum that is involved in generation of psychomotor behavior. Our experiments demonstrate heterogeneity of S cell populations along the rostrocaudal axis in terms of RARβ signaling. The population of late-born S cells, which is preferentially expanded by high levels of RA through RARβ signaling to form a large S compartment in the rostral striatum, may engage in modulating neural circuits of psychomotor function.
Regulation of Proliferation of S Progenitors by RARβ Signaling.
RARβ mutation resulted in defective proliferation of late-born S cells. In addition to the differentiated mantle zone, RARβ is expressed at low levels in the progenitor domains of LGE (Fig. S5) and by progenitor cells in neurospheres derived from striatal anlage (18). The expression of RARβ in striatal progenitors suggests a cell-autonomous effect of RARβ in regulating proliferation of striatal progenitors, which is corroborated by our finding that RA increased proliferation of striatal progenitors-derived ST14A cells. A previous cell lineage study has suggested a heterogeneity of S and M progenitors within the proliferative VZ (24). It is likely that both the late-born S progenitors and the M progenitors were concurrently labeled by the 1-hour pulse of BrdU at E12.75 LGE. Because the population of M cells is the dominant population in the striatum (≈80–85% of total striatal neurons) and the neurogenesis of M cells was not affected by RARβ mutation, the RARβ mutation-induced moderate decreases in cell proliferation and Ccne2 and Mash1 mRNA levels are likely to reflect a selectivity of defective neurogenesis of the small population of late-born S progenitors. The presence of low levels of RARβ in the progenitor domains may thus suggest a selective expression of RARβ in a small population of S progenitors.
A Stage-Dependent Dual Mode of RA Signaling for Controlling Striatal Development.
It is unclear why the proliferation of M progenitor cells was unaffected in RARβ−/− mutant LGE, which may be due to the absence of RARβ or selective expression of RA degrading enzymes in M progenitors. Unlike M progenitor cells, differentiating M cells were competent for transducing RA signals, because Ebf-1, a differentiating marker of M cells (14), was increased in RARβ−/− striatum, suggesting that differentiation of M cells is regulated by RARβ signaling. RA signaling has been shown to regulate differentiation of striatal molecules, particularly the molecules involved in dopamine signal transduction including D1 and D2 receptors and DARPP-32 (6, 10, 12, 25–28). Given that most RA-regulated dopamine signaling molecules are first expressed by S and also later by M cells during development, we propose a stage-dependent dual mode of RA signaling in which RA first regulates proliferation of late-born S progenitors and later differentiation of postmitotic S and M cells in striatal development.
Patterning the Labyrinthine of Striatal Compartments by RARβ Signaling.
The S compartments, as shown by 3D reconstruction study, form a labyrinthine structure in which the S compartment is larger and more elaborate at the rostral than the middle and caudal levels (29). Given the differentially proliferating effects by RA on S cells undergoing neurogenesis at different time windows, we propose a working model that RARβ signaling plays a crucial role in setting up the population sizes of S compartments along the rostrocaudal axis (Fig. 6). Under physiological conditions, the early-born S cells, due to deficiency of RA at their neurogenesis at E10.5–E11.5, develop into a small S compartment in the caudal striatum. Subsequently, elevated concentration of RA at E12.5–E13.5 LGE may enable full activation of RARβ signaling in late-born S cells, which leads to preferential expansion of the late-born S cell population. The late-born S cell population eventually evolves to form a fully blown S compartment in the rostral striatum.
Fig. 6.
Schematic drawings illustrating the working hypothesis of modular patterning of striatal compartments by RARβ signaling. The early-born S cells, because of deficiency of endogenous RA in the LGE during their neurogenesis at E10.5–E11.5, develop into a small S compartment in the caudal striatum. Subsequently elevated RA at E12.5–E13.5 enables full activation of RARβ signaling in late-born S cells, which leads to preferential expansion of the late-born S cell population. The expanded S cell population eventually evolves to form a large and elaborate S compartment in the rostral striatum, which may modulate psychomotor function. Note that, for simplicity, the effects of RA signaling on promoting differentiation of striatal neurons are omitted from the drawings.
Potential Involvement of the Rostral S Compartment in Neural Circuits of Psychomotor Function.
The enlarged S compartment by RARβ signaling in the rostral striatum should presumably have topographically functional significance. Challenging the RARβ−/− mutant mice with apomorphine revealed a differentially responsive profile of various stereotypic behaviors, including exaggeration of head-bobbing movement and reduction of rearing activity. These behavioral phenotypes were unlikely due to changes of dopamine receptors in the mutant striatum, because D1 and D2 receptors are not altered in RARβ−/− mutant mice (12). The behavioral phenotypes instead imply that neurons in the rostral S compartment may modulate complex neural circuits of psychomotor control. It has been proposed that unbalanced activity shifting toward increased neuronal activity in S compartment is correlated with motor stereotypic behavior (30, 31), although another study has argued that motor stereotype does not require enhanced activity in S neurons (32). The S compartment receives limbic system-associated inputs from the cerebral cortex and projects to dopaminergic neurons of the pars compacta of the substantia nigra, which may provide feedback control of dopaminergic inputs to striatal circuits (2, 3). The loss of rostral S compartment in RARβ−/− mutant striatum would conceivably result in aberrant neural circuits and perhaps change of dopamine tone within the basal ganglia, which in turn leads to altered drug-induced repetitive motor behavior. We cannot, however, rule out the possibility that there may be some uncharacterized defects in other brain regions that contribute to the behavioral phenotype of RARβ−/− mutant mice.
Aberrant RA Signaling and the Pathogenesis of Psychomotor Disorders.
Clinical relevance of RA signaling in regulation of stereotypic motor behavior has been reported in patients with obsessive-compulsive disorder (OCD), because the stereotypic motor symptoms of OCD patients are alleviated with RA treatments (33). Autism, a neurodevelopmental disorder characterized by aberrant repetitive behavior, is linked to defective corticobasal ganglia circuits (34). A recent MRI study has reported a decrease in the volume of the caudate nucleus in patients with autism (35), and the etiology of autism may be associated with abnormal RA signaling and MOR deficiency (36, 37). Clinical case reports also implicate potential RA treatments for autism (38). Taken together, given that MOR1 is selectively expressed in striatal S compartments, our findings of RARβ mutation-induced aberrant S compartments and associated psychomotor dysfunction may have clinical implication for understanding the pathogenesis of autism and other psychomotor disorders.
Experimental Procedures
Detailed experimental procedures are described in SI Text.
Mutant Mice.
RARβ mutant mice were generated (39). See Experimental Procedures in SI Text.
Immunohistochemistry.
Immunohistochemistry was performed as described (40) with the following primary antibodies: rabbit MOR1 [1:10,000, gift of R. P. Elde (University of Minnesota, Minneapolis)] mouse calbindin (1:500, Sigma), rabbit met-enkephalin (1:2,000, gift of R. P. Elde), rabbit DARPP-32 (1:150, Cell Signaling), rabbit tyrosine hydroxylase (1:2,000, EugenTech), rabbit Ki67 (1:200, NovoCastra), rabbit βIII-tubulin (TuJ1, 1:1,000, Covance), sheep BrdU (1:200, Biodesign) and rabbit GluR1 (1:200, Upstate Biotechnology).
In Situ Hybridization.
In situ hybridization was performed as described (13). See Experimental Procedures in SI Text.
Cell Culture.
The cultivation of explant tissue, Sil-15 RA reporter cells, and ST14 cells was performed as described (9, 20, 23). See Experimental Procedures in SI Text.
Behavioral Tests.
See Experimental Procedures in SI Text.
Supplementary Material
Acknowledgments.
We thank R. P. Elde and E. Cattaneo (University of Milan, Milan) for reagents, D.-Y. Chen for advice on statistics, and C. P. Hung for reading the manuscript. This work was funded in part by National Health Research Institutes (Grants EX94, EX95, EX96, EX97-9402NI), National Research Program for Genomic Medicine (Grants NSC95-3112-B-010-014, NSC96-3112-B-010-007), and Ministry of Education (Aim for Top University Grant, 96A-D-T171) in Taiwan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802109105/DCSupplemental.
References
- 1.Graybiel AM, Ragsdale CW., Jr Histochemically distinct compartments in the striatum of human, monkey, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 3.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 4.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: Lessons from Genetic and Pharmacological Dissections of the Retinoic Acid Signaling Pathway During Mouse Embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 5.Maden M. Retinoid signalling in the development of the central nervous system. Nat Rev Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- 6.Toresson H, Mata de Urquiza A, Fagerstrom C, Perlmann T, Campbell K. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development. 1999;126:1317–1326. doi: 10.1242/dev.126.6.1317. [DOI] [PubMed] [Google Scholar]
- 7.Smith D, Wagner E, Koul O, McCaffery P, Drager UC. Retinoic acid synthesis for the developing telencephalon. Cereb Cortex. 2001;11:894–905. doi: 10.1093/cercor/11.10.894. [DOI] [PubMed] [Google Scholar]
- 8.Marklund M, Sjodal M, Beehler BC, Jessell TM, Edlund T, Gunhaga L. Retinoic acid signalling specifies intermediate character in the developing telencephalon. Development. 2004;131:4323–4332. doi: 10.1242/dev.01308. [DOI] [PubMed] [Google Scholar]
- 9.Liao WL, et al. Retinoid signaling competence and RARbeta-mediated gene regulation in the developing mammalian telencephalon. Dev Dyn. 2005;232:887–900. doi: 10.1002/dvdy.20281. [DOI] [PubMed] [Google Scholar]
- 10.Molotkova N, Molotkov A, Duester G. Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev Biol. 2007;303:601–610. doi: 10.1016/j.ydbio.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolle P, Fraulob V, Kastner P, Chambon P. Developmental expression of murine retinoid X receptor (RXR) genes. Mech Dev. 1994;45:91–104. doi: 10.1016/0925-4773(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 12.Krezel W, et al. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- 13.Liao WL, Tsai HC, Wu CY, Liu FC. Differential expression of RARbeta isoforms in the mouse striatum during development: a gradient of RARbeta2 expression along the rostrocaudal axis. Dev Dyn. 2005;233:584–594. doi: 10.1002/dvdy.20344. [DOI] [PubMed] [Google Scholar]
- 14.Garel S, Marin F, Grosschedl R, Charnay P. Ebf1 controls early cell differentiation in the embryonic striatum. Development. 1999;126:5285–5294. doi: 10.1242/dev.126.23.5285. [DOI] [PubMed] [Google Scholar]
- 15.Graybiel AM, Hickey TL. Chemospecificity of ontogenetic units in the striatum: demonstration by combining [3H] thymidine neuronography and histochemical staining. Proc Natl Acad Sci USA. 1982;79:198–202. doi: 10.1073/pnas.79.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Kooy D, Fishell G. Neuronal birthdate underlies the development of striatal compartments. Brain Res. 1987;401:155–161. doi: 10.1016/0006-8993(87)91176-0. [DOI] [PubMed] [Google Scholar]
- 17.Song DD, Harlan RE. Genesis and migration patterns of neurons forming the patch and matrix compartments of the rat striatum. Dev Brain Res. 1994;83:233–245. doi: 10.1016/0165-3806(94)00144-8. [DOI] [PubMed] [Google Scholar]
- 18.Wohl CA, Weiss S. Retinoic acid enhances neuronal proliferation and astroglial differentiation in cultures of CNS stem cell-derived precursors. J Neurobiol. 1998;37:281–290. doi: 10.1002/(sici)1097-4695(19981105)37:2<281::aid-neu7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Lauper N, et al. Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene. 1998;17:2637–2643. doi: 10.1038/sj.onc.1202477. [DOI] [PubMed] [Google Scholar]
- 20.Cattaneo E, Conti L. Generation and characterization of embryonic striatal conditionally immortalized ST14A cells. J Neurosci Res. 1998;53:223–234. doi: 10.1002/(SICI)1097-4547(19980715)53:2<223::AID-JNR11>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Li H, et al. A retinoic acid synthesizing enzyme in ventral retina and telencephalon of the embryonic mouse. Mech Dev. 2000;95:283–289. doi: 10.1016/s0925-4773(00)00352-x. [DOI] [PubMed] [Google Scholar]
- 22.McCaffery P, Drager UC. High levels of a retinoic-acid generating dehydrogenase in the meso-telencephalic dopamine system. Proc Natl Acad Sci USA. 1994;91:7772–7776. doi: 10.1073/pnas.91.16.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner W, Han B, Jessell TM. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development. 1992;116:55–66. doi: 10.1242/dev.116.1.55. [DOI] [PubMed] [Google Scholar]
- 24.Krushel LA, Johnston JG, Fishell G, Tibshirani R, van der Kooy D. Spatially localized neuronal cell lineages in the developing mammalian forebrain. Neuroscience. 1993;53:1035–1047. doi: 10.1016/0306-4522(93)90487-z. [DOI] [PubMed] [Google Scholar]
- 25.Samad TA, Krezel W, Chambon P, Borrelli E. Regulation of dopaminergic pathways by retinoids: activation of the D2 receptor promoter by members of the retinoic acid receptor- retinoid X receptor family. Proc Natl Acad Sci USA. 1997;94:14349–14354. doi: 10.1073/pnas.94.26.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdenaire O, Maus-Moatti M, Vincent JD, Mallet J, Vernier P. Retinoic acid regulates the developmental expression of dopamine D2 receptor in rat striatal primary cultures. J Neurochem. 1998;71:929–936. doi: 10.1046/j.1471-4159.1998.71030929.x. [DOI] [PubMed] [Google Scholar]
- 27.Liao WL, Liu FC. RARbeta isoform-specific regulation of DARPP-32 gene expression: an ectopic expression study in the developing rat telencephalon. Eur J Neurosci. 2005;21:3262–3268. doi: 10.1111/j.1460-9568.2005.04178.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang H-F, Liu F-C. Regulation of multiple dopamine signal transduction molecules by retinoids in the developing striatum. Neuroscience. 2005;134:97–105. doi: 10.1016/j.neuroscience.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Desban M, Kemel ML, Glowinski J, Gauchy C. Striatal organization of patch and matrix compartments in the rat striatum. Neuroscience. 1993;57:661–671. doi: 10.1016/0306-4522(93)90013-6. [DOI] [PubMed] [Google Scholar]
- 30.Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- 31.Saka E, Goodrich C, Harlan P, Madras BK, Graybiel AM. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci. 2004;24:7557–7565. doi: 10.1523/JNEUROSCI.1072-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glickstein SB, Schmauss C. Focused motor stereotypies do not require enhanced activation of neurons in striosomes. J Comp Neurol. 2004;469:227–238. doi: 10.1002/cne.11000. [DOI] [PubMed] [Google Scholar]
- 33.Gupta MA, Schork NJ, Ellis CN. Psychosocial correlates of the treatment of photodamaged skin with topical retinoic acid: a prospective controlled study. J Am Acad Dermatol. 1994;30:969–972. doi: 10.1016/s0190-9622(94)70119-9. [DOI] [PubMed] [Google Scholar]
- 34.Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2006;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAlonan GM, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- 36.London E, Etzel RA. The environment as an etiologic factor in autism: a new direction for research. Environ Health Perspect. 2000;3(108 Suppl):401–404. doi: 10.1289/ehp.00108s3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 38.Megson MN. Is autism a G-alpha protein defect reversible with natural vitamin A? Med Hypotheses. 2000;54:979–983. doi: 10.1054/mehy.1999.0999. [DOI] [PubMed] [Google Scholar]
- 39.Ghyselinck NB, et al. Role of the retinoic acid receptor beta (RAR beta) during mouse development. Int J Dev Biol. 1997;41:425–447. [PubMed] [Google Scholar]
- 40.Wang H-F, Liu F-C. Developmental restriction of the LIM homeodomain transcription factor Isl-1 expression to cholinergic neurons in the striatum. Neuroscience. 2001;103:999–1016. doi: 10.1016/s0306-4522(00)00590-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.